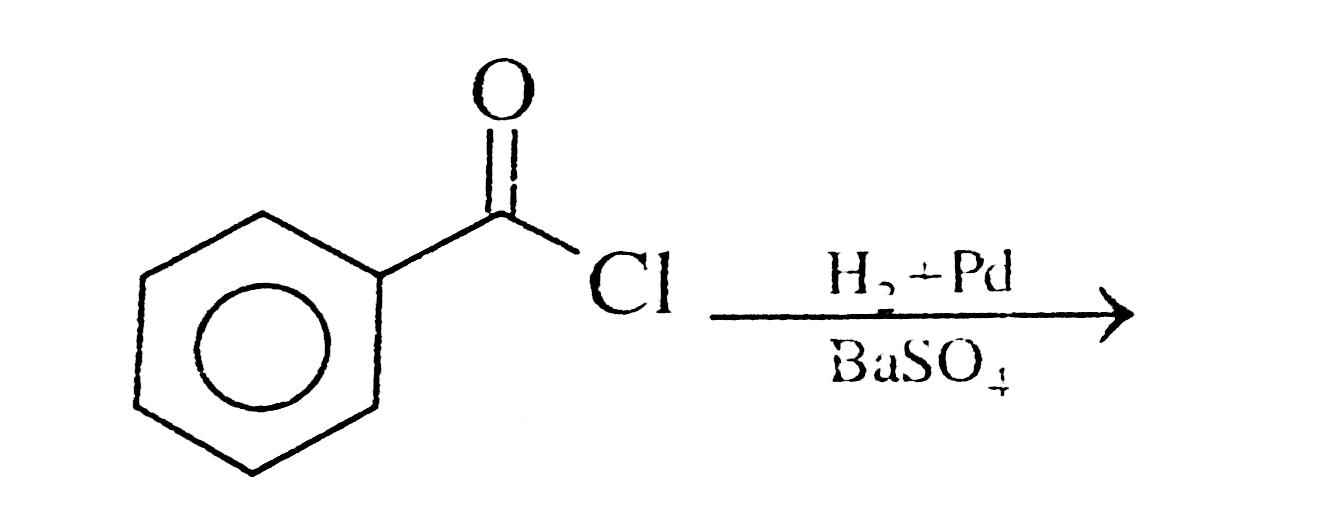
…………….. reaction is also known as a substitution reaction.
Step-by-Step Solution:
1. Understanding the Question:
The question asks for a type of reaction that is also known as a substitution reaction.
2. Defining Substitution Reaction:
A substitution reaction is defined as a chemical reaction in which one functional group or atom is replaced by another functional group or atom.
3. Key Characteristics of Substitution Reactions:
- A more reactive functional group or atom can replace a less reactive one.
- Conversely, a less reactive functional group or atom cannot replace a more reactive one.
4. Examples of Substitution Reactions:
- Example 1: When methane (CH4) reacts with chlorine (Cl2) in the presence of sunlight, one hydrogen atom in methane is replaced by a chlorine atom, forming chloromethane (CH3Cl) and hydrochloric acid (HCl). This is a substitution reaction.
- Example 2: When zinc (Zn) is placed in copper sulfate (CuSO4) solution, zinc displaces copper, forming zinc sulfate (ZnSO4) and copper metal. This is another example of a substitution reaction.
5. Identifying the Type of Reaction:
Substitution reactions are also referred to as single displacement reactions.
6. Final Answer:
Therefore, the reaction that is also known as a substitution reaction is called a single displacement reaction.
---
The question asks for a type of reaction that is also known as a substitution reaction.
A substitution reaction is defined as a chemical reaction in which one functional group or atom is replaced by another functional group or atom.
- A more reactive functional group or atom can replace a less reactive one.
- Conversely, a less reactive functional group or atom cannot replace a more reactive one.
- Example 1: When methane (CH4) reacts with chlorine (Cl2) in the presence of sunlight, one hydrogen atom in methane is replaced by a chlorine atom, forming chloromethane (CH3Cl) and hydrochloric acid (HCl). This is a substitution reaction.
- Example 2: When zinc (Zn) is placed in copper sulfate (CuSO4) solution, zinc displaces copper, forming zinc sulfate (ZnSO4) and copper metal. This is another example of a substitution reaction.
Substitution reactions are also referred to as single displacement reactions.
Therefore, the reaction that is also known as a substitution reaction is called a single displacement reaction.
टॉपर्स ने हल किए ये सवाल
- CHEMICAL REACTIONS BOOK - ICSECHAPTER - CHEMICAL REACTIONS EXERCISE - EXERCISES- D. MATCH THE FOLLOWING
5 Videos
- CHEMICAL REACTIONS BOOK - ICSECHAPTER - CHEMICAL REACTIONS EXERCISE - EXERCISES- G. GIVE REASONS FOR THE FOLLOWING
4 Videos
- CHEMICAL REACTIONS BOOK - ICSECHAPTER - CHEMICAL REACTIONS EXERCISE - EXERCISES- B. TRUE OR FALSE
5 Videos
- CARBON AND ITS COMPOUNDS BOOK - ICSECHAPTER - CARBON AND ITS COMPOUNDS EXERCISE - EXERCISES (E NAME THE FOLLOWING)
10 Videos
- ELEMENTS , COMPOUNDS AND MIXTURES BOOK - ICSECHAPTER - ELEMENTS , COMPOUNDS AND MIXTURES EXERCISE - Think and answer
3 Videos
समान प्रश्न
ज्ञान जांच
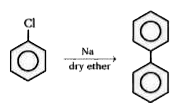 Above reaction is known as:AWurtz reactionBFittig reactionCWurtz fittig reactionDKolbe electrolysis
Above reaction is known as:AWurtz reactionBFittig reactionCWurtz fittig reactionDKolbe electrolysis
This reaction is known as:AClaisen rearrangementBTischenko reactionCPerkin reactionDCannizzaro reaction
The above reaction is known as:ASchmidt reactionBHofinann rearrangementCCurtius reactionDLossen rearrangement
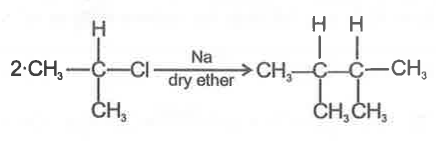 The above reaction is known as
The above reaction is known as
View SolutionThe given reaction
 is known as :
is known as :
View SolutionWhich one of the following reaction is an example of free radical substitution reaction?
View SolutionAssertion : Aniline does not undergo Friendel-Crafts reaction .
Reason : Friedel-Craft reaction is an electrophilic substitution reaction .
View SolutionRate of substitution reaction in phenol is :
View Solution