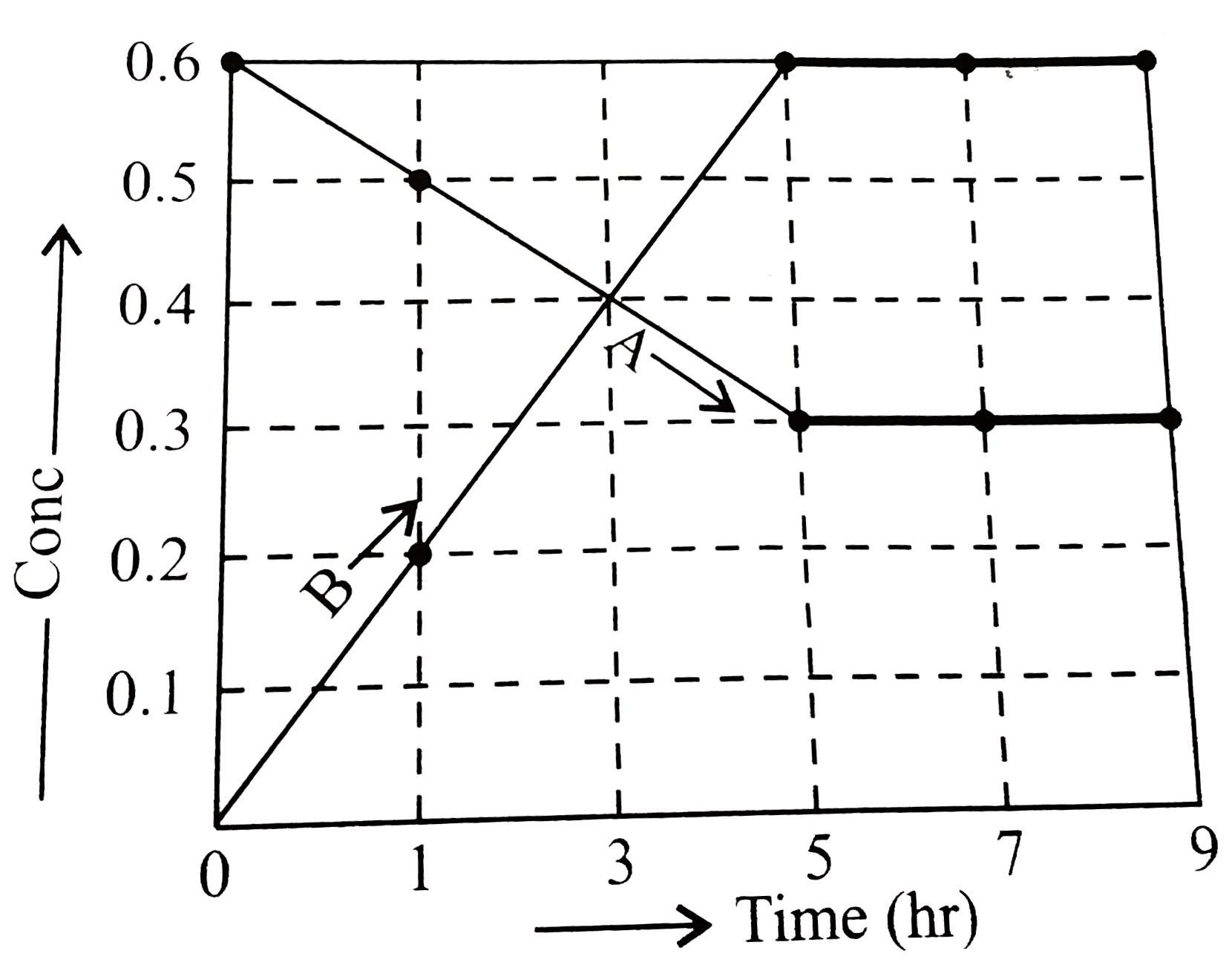
The progress of the reaction A⇔nB , with time is presented in the figure. Determine (i) the value of n (ii) the equilibrium constant K (iii) the initial rate of conversion of A

At equilibrium Rate (forward) = Rate (backward)
=0.6−0.35=0.65×1n
For the eqb: A⇔2B,K=0.620.3
Initial rate = change in concentration of A in the 1st hour
2,1.2,0.1
Topper's Solved these Questions
Similar Questions
The progress of the reaction, A ⇌ nB with time, is presented in figure given below. Determine.
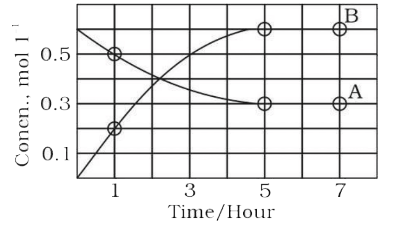
(i) The value of n,
(ii) The equilibrium constant K, and
(iii) the initial rate of conversion of A.
View SolutionThe progress of the reaction A⇔nB with time is persented in the figure given below:
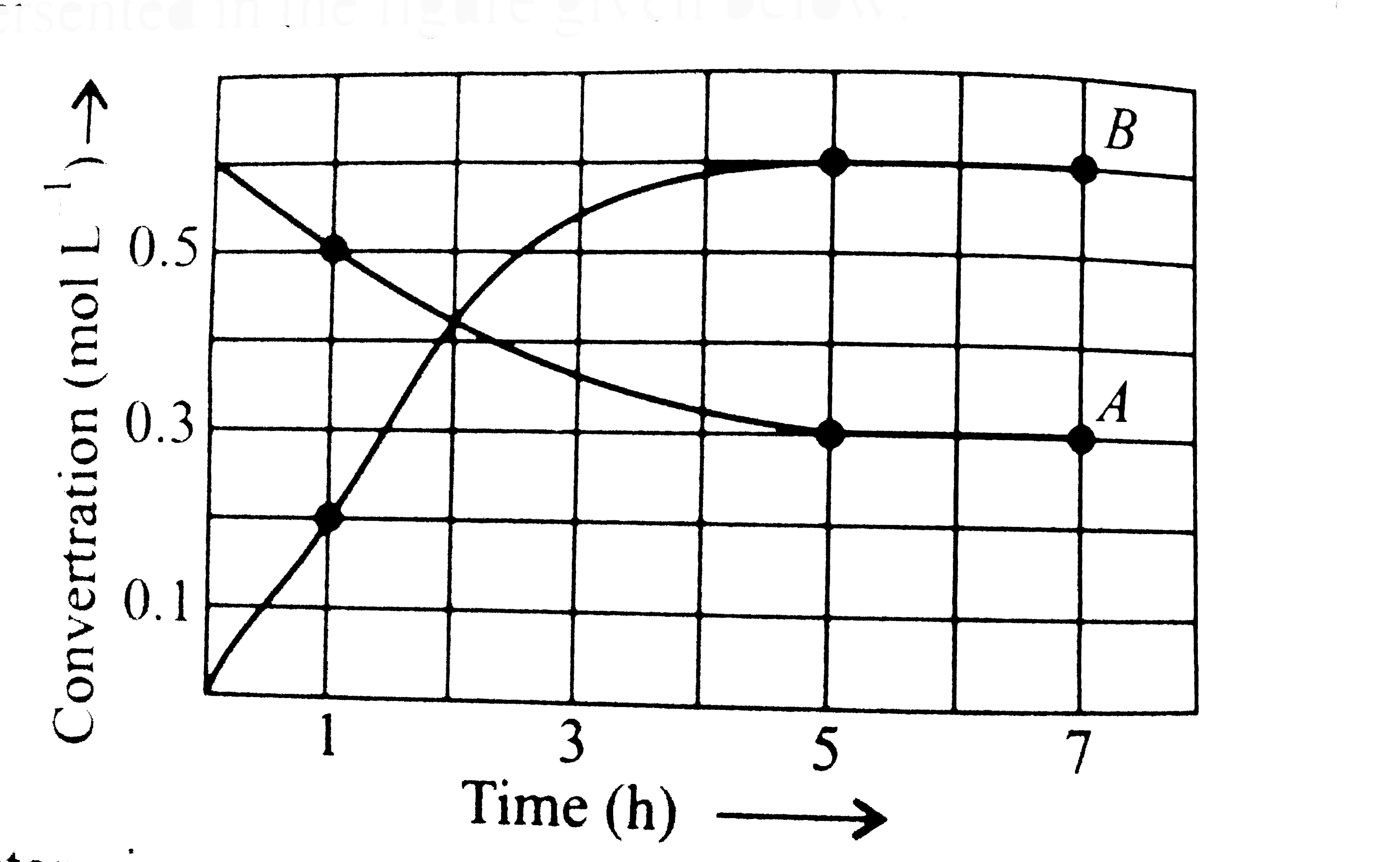
Determine
a. The value of n.
b. The equilibrium constant K.
c. The initial rate of concentration of A.
View Solution
Knowledge Check
The process of the reaction A⇔nB with time is represented in the fig, given below
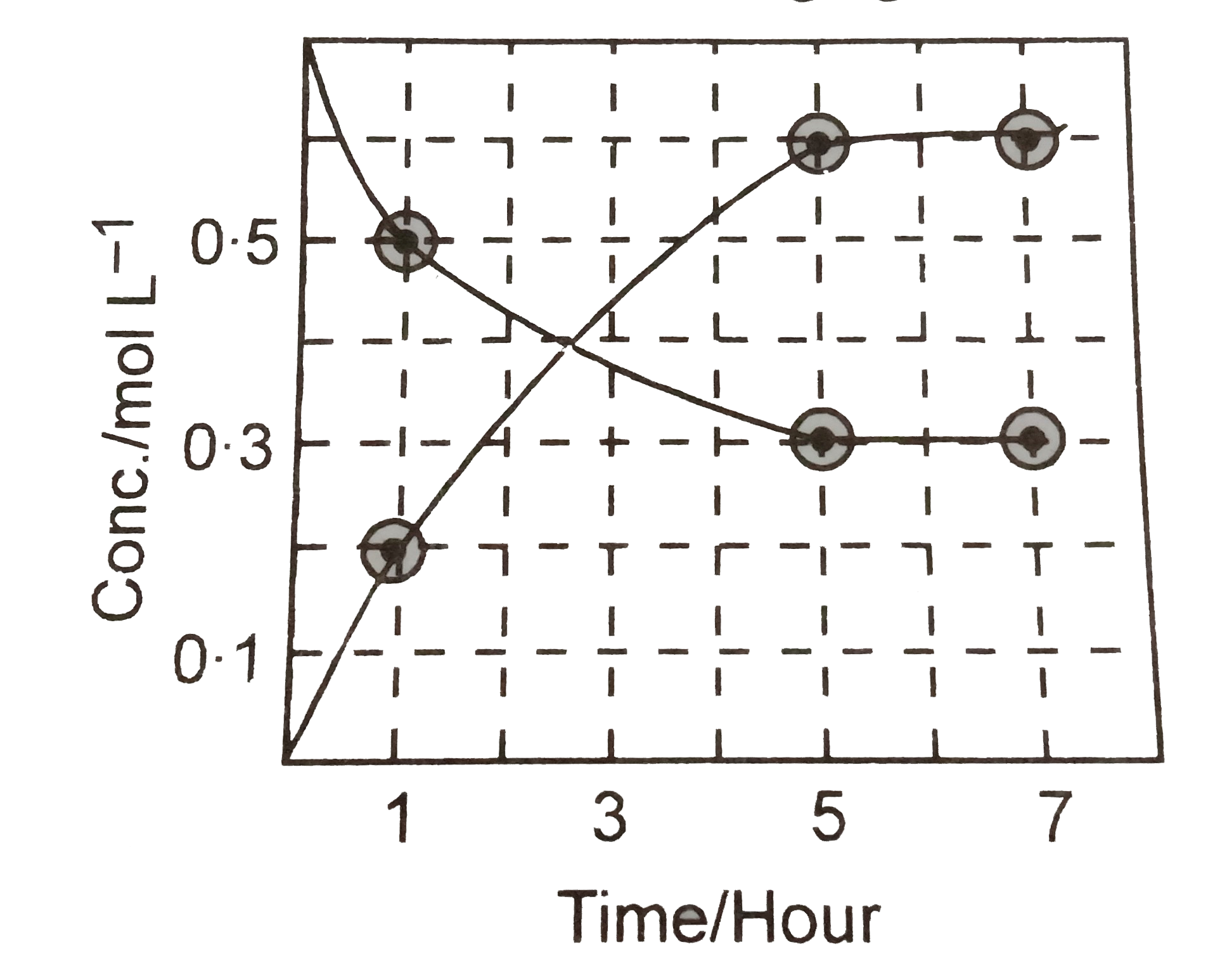
The value of n isA1B2C3D1.5
The progress of the reaction A⇔nB with time, is presented in figure. Determine
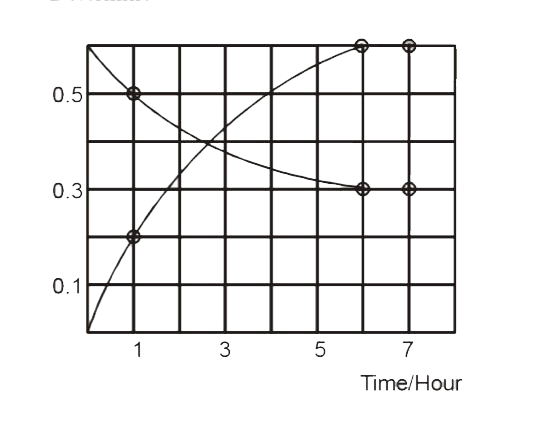
the value of n.
View SolutionThe progress of the reaction A⇔nB with time, is presented in figure. Determine
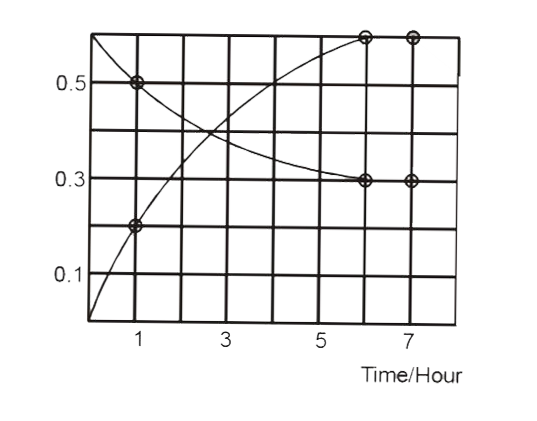
the equilibrium constant K.
View SolutionThe progress of the reaction A⇔nB with time, is presented in figure. Determine
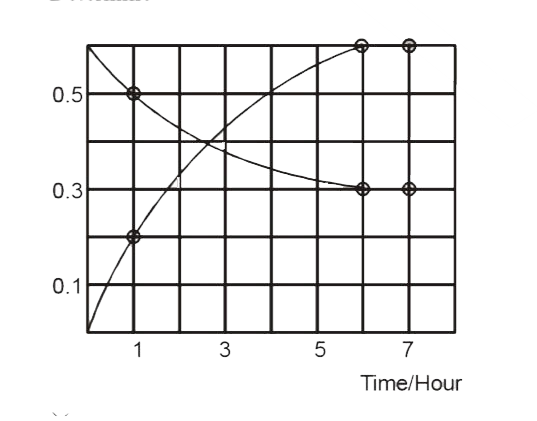
the initial rate of conversion of A.
View SolutionThe progress of the reaction A⇔nB, with times is presented in the figure below.
Determine:
(a) The value of n , (b) Keq
( c) Initial rate of converison of A
View SolutionDetermine the values of equilibrium constant (KC)andΔGo for the following reaction :
View SolutionThe gaseous reaction
2A(g)+nB(g)⇔mC(g) is represented by following curves.
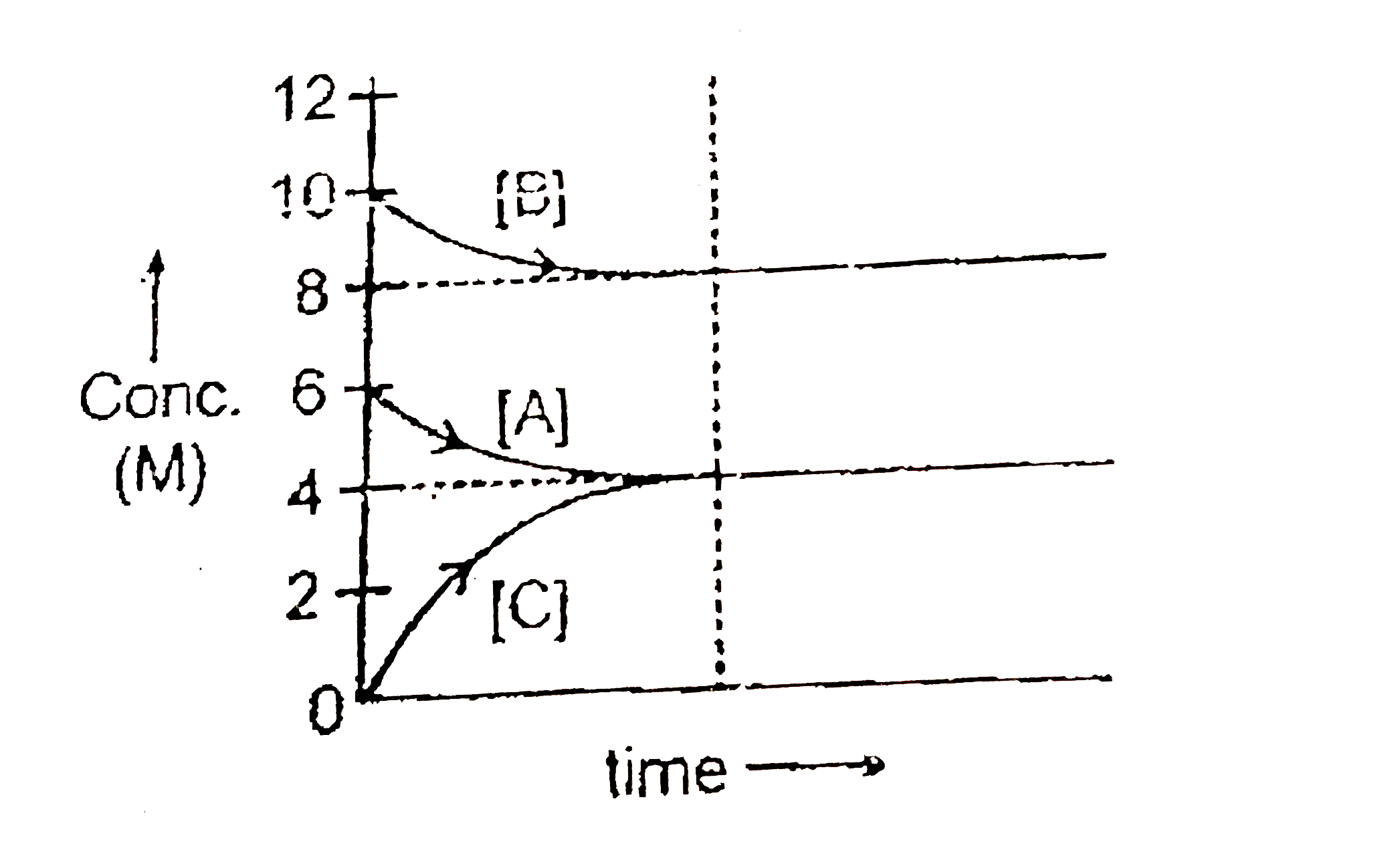
What is the value of m+n?
View SolutionWhat will be the value of ΔG .If equilibrium constant for a reaction is 10 at 300K
View Solution

