A proposed mechanism for the catalysed decomposition of aqueous H2O2 is
H2O2+I−k1−−→H2O+IO−
H2O2+IO−k2−−→H2O+O2+I−
where k2>>k1 . Derive the rate law for the reaction.
−d[H2O2]dt=k1[H2O2][I−]
Topper's Solved these Questions
Similar Questions
Consider the decomposition of hydrogen peroxide in alkaline medium which is catalysed by iodide ions.
2H2O2OH−−−→2H2O+O2
This reaction takes place in two steps as given below :
Step I H2O2+I−→H2O+IO− (slow )
Step II H2O2+IO−→H2O+I−+O2 ( fast )
(a) Write the rate law expression and determine the order of reaction w.r.t. H2O2.
(b) What is the molecularity of each individual step ?
View SolutionThe possible mechanism for the reaction
2NO+2H2→N2+2H2O is
i) 2NO<⇒N2O2
ii) N2O2+H2slow−−→N2O+H2O
iii) N2O+H2fast−−→N2+H2O
a) What is rate law for the reaction?
b) What is the order of the reaction?
View Solution
Knowledge Check
H2O2R.T.−−→H2O+12O2
AFor disproportionation reaction.BFor comproportionation reaction.CFor either intermolecular redox reaction or displacement reactionDFor either thermal combination redox reaction or thermal decomposition redox reaction.H2O2R.T.−−→H2O+12O2
AFor disproportionation reaction.BFor comproportionation reaction.CFor either intermolecular redox reaction or displacement reactionDFor either thermal combination redox reaction or thermal decomposition redox reaction.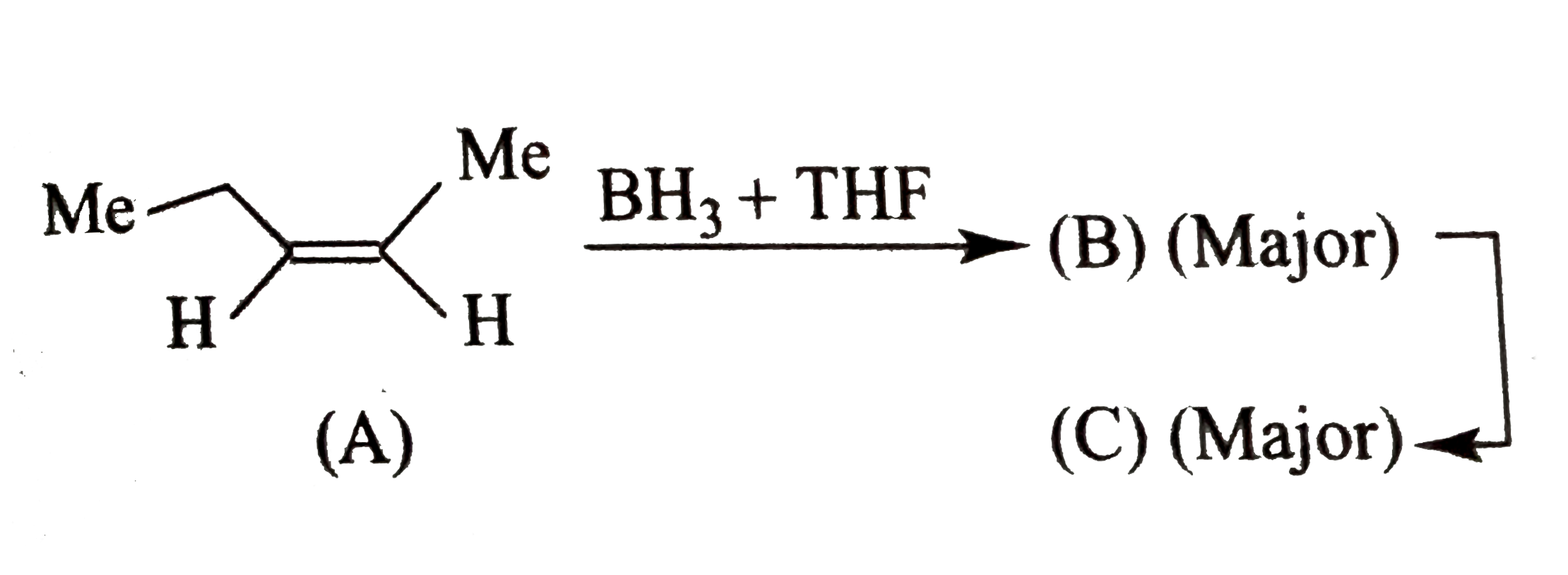 H2O2+ΘOHA
H2O2+ΘOHA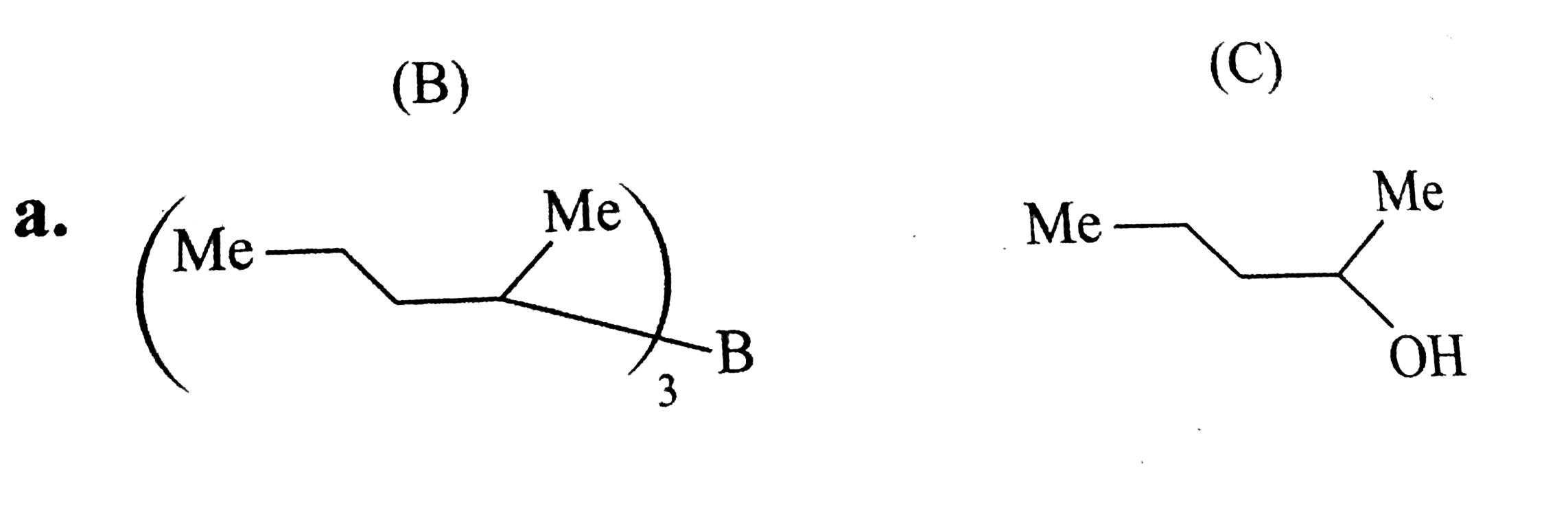 B
B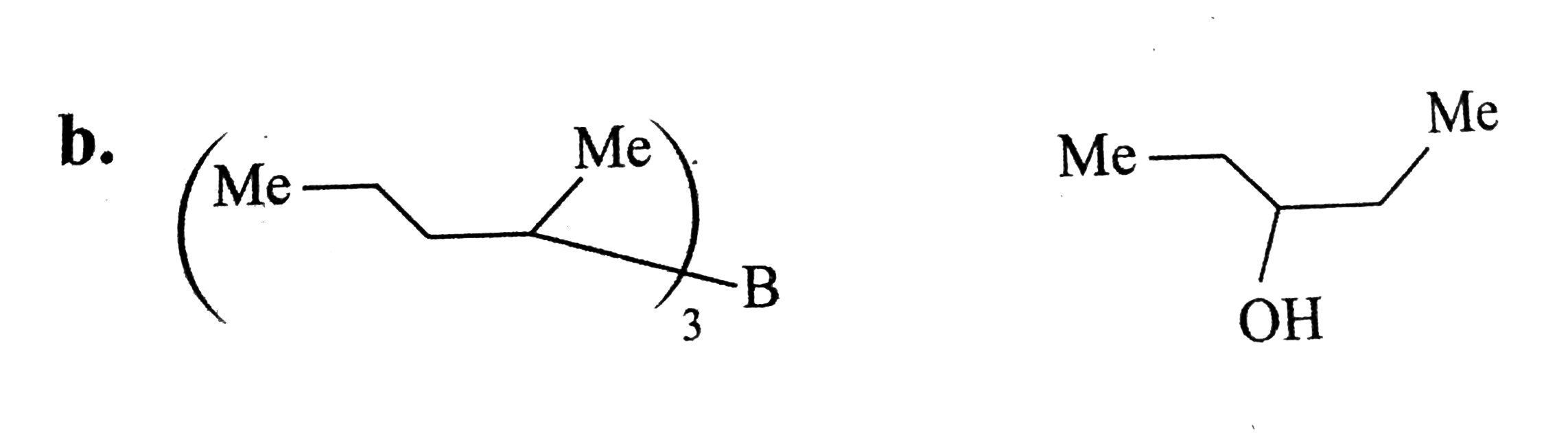 C
C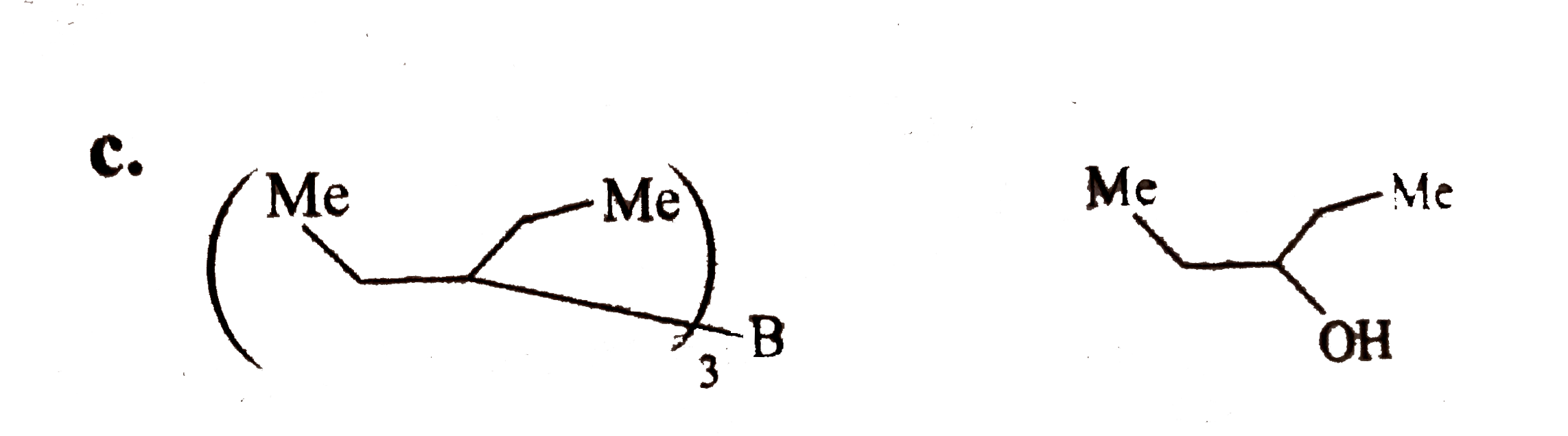 D
D
Consider the decomposition of hydrogen peroxide in the alkaline medium which is catalysed by iodide ions.
This reaction takes place in two steps as given below
step-1 H2O2+I−1→H2O+IO−(Slow)
Step II H2O2+IO−→H2O+I−+O2 (fast)
a) Write the rate law expression and determine the order of reactan w.r.t H2O2
b) What is the molecularity of each individual step?
View SolutionConsider the decomposition reaction :
2H2O2OH−/I−−−−−−−→2H2O+O2
This reaction takes place in two steps as given below :
Step 1. H2O2+I−→H2O+IO− (slow)
Step 2. H2O2+IO−→H2O+I−+O2 (fast)
Determine the order of reaction.
View SolutionCaC2+H2O→XO3/H2O/OH+−−−−−−−−−→HCOOH X is
View SolutionFor a reaction, 2NO+2H2→N2+2H2O, the possible mechanism is
NO⇔N2O2
N2O2+H2Slow−−→N2O+H2O
N2O+H2Ofast−−→N2+H2O
What is the rate law and order of the reaction?
View SolutionThe mechanism of the reaction
2NO+O2→2NO2 is
NO+NOk1⇐⇒k−1N2O2(fast)
N2O2+O2k2−−→2NO2(slow)
The rate constant of the reaction is
View Solution
