An organic compound A (C12H20) decolarizes bromine water, and on reductive ozonolysis produces two molecules of B (C6H10O). Compound B on aldol condensation followed by dehydration of product gives C (C12H18O), which on reduction with N2H4/OH2 regenerates A. COmpound B on reduction with Zn(Hg)HCl produces D (C6H12), which on monochlorination gives E (C6H11Cl) as the sole product.




The correct Answer is:D
Topper's Solved these Questions
- ALDEHYDES AND KETONES II. ALDOL REACTIONS Book:MS CHOUHANChapter:ALDEHYDES AND KETONES II. ALDOL REACTIONS Exercise:ADDITIONAL OBJECTIVE QUESTIONS ( SINGLE CORRECT CHOICE TYPE)
Explore 13 Videos
- ALDEHYDES AND KETONES I. NUCLEOPHILIC ADDITION TO THE CARBONYL GROUPBook:MS CHOUHANChapter:ALDEHYDES AND KETONES I. NUCLEOPHILIC ADDITION TO THE CARBONYL GROUPExercise:ADDITIONAL OBJECTIVE QUESTIONS (SINGLE CORRECT CHOICE TYPE)
Explore 17 Videos
- ALDOL AND CANNIZARO REACTIONBook:MS CHOUHANChapter:ALDOL AND CANNIZARO REACTIONExercise:Subjective Problems
Explore 3 Videos
Similar Questions
Mixed aldol reactions require
View SolutionHydrogenation of benzoyl chloride in the presence of Pd and BaSO4 gives
View Solution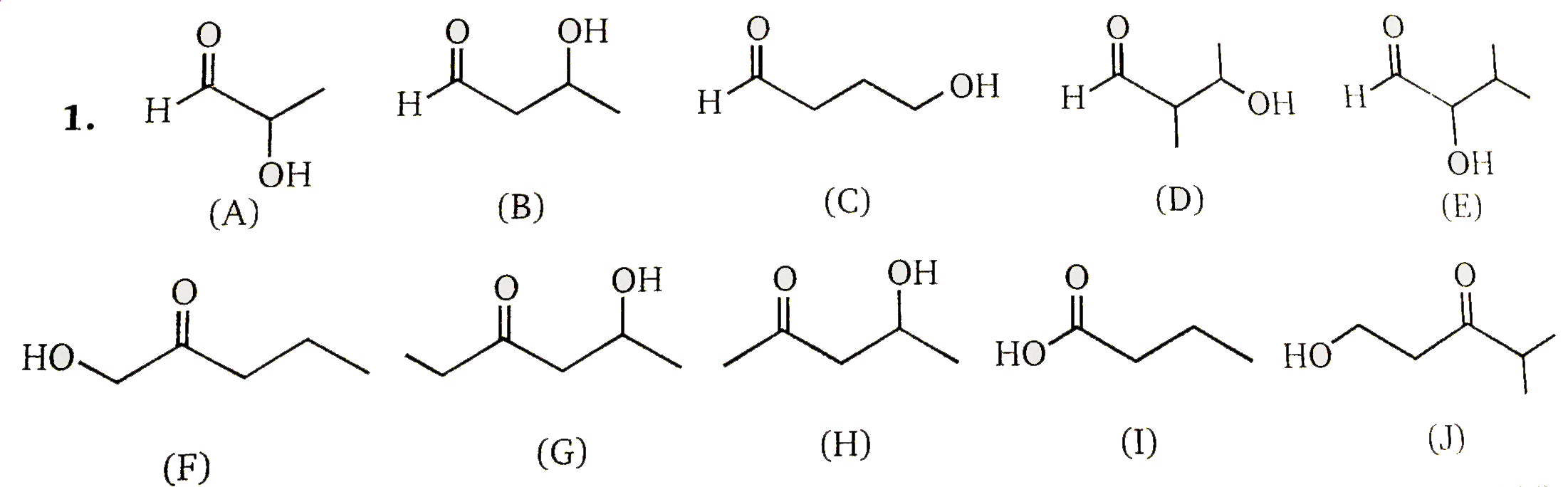
X=number of compound obtained by aldol reaction
Y=Number of compounds react with NaHCO3
Sum of X+Y is
View SolutionAn organic compound (A) C_(6)H_(10) on reduction, first give (B) C_(6)H_(12) and finally (C) C_(6)H_(14) (A) on reaction with ozone followed by hydrolysis is presence of Zn gives two aldehydes C_(2)H_(4)O (D) and C_(2)H_(2)O_(2) (E) Oxdation of (B) with acidified KMnO_(4) gives acid (F) C_(3)H_(6)O_(2) Determine structures of (A) to (F) with proper reasoning.
View SolutionCompound (A) C12H20 discharges the colour of Br2−H2O and cold KMnO4 . On reduction with H2/Pt it gives compound (B) C12H22 . A on ozonolysis give cyclohexanone . Find structure of A :
View SolutionFind DU of following compound:-
(a) C6H6O , (b)C6H5I , ( c)C6H6ClBrO , (d)C5H9N
View SolutionOn ozonolysis, an organic compound (A) C6H10 gives two aldehyde (B) C2H4O and (C ) C2H2O2. Determine the structure of A,B and C.
View SolutionA hydrocarbon A(C_(10)H_(12)) has no chiral carbon. A gives a white precipitate with ammonical solution of silver nitrate. A on treatment with H_(2)//pt gives B(C_(10)H_(20)) . A on ozonolysis gives C(C_(8)H_(12)O_(4)) as one product which on heating with soda lime gives D(C_(6)H_(12)) . D on monochlorination with Cl_(2)//hv gives C_(6)H_(11)Cl as sole isomer. Identify A to D .
View SolutionAn organic compound [A] C6H10, on reduction first gives [B] C6H12, and finally [C] C6H14. [A] on ozonolysis followed by hydrolysis gives two aldehydes [D] C2H4O, and [E] C2H2O. Oxidation of [B] with acidified KMnO4 gives acid [F] C4H8O2. Determine the structures of the compounds [A] to [F] with proper reasoning.
View SolutionC2H6O सूत्र वाले कार्बनिक यौगिक (A) को सांद्र H2SO4 के साथ 413 k गर्म करने पर C4H10O सूत्र वाला उदासीन यौगिक (B) प्राप्त होता है। (B) की क्रिया PCl5 से कराने पर एक उत्पाद प्राप्त होता है जिसकी क्रिया KCN के साथ कराने पर C3H5N सूत्र वाला यौगिक (C) प्राप्त होता है। यौगिक (C) जल-अपघटन पर एक अम्ल C3H6O2 देता है तथा Na/Hg द्वारा अपचयन पर एक क्षारक C3H9N देता है। यौगिक (A), (B) तथा (C) क्या हैं? सन्निहित अभिक्रियाएँ भी दीजिए।
View Solutionअणुसूत्र C3H6O युक्त एक कार्बनिक यौगिक ( A) फेहलिंग विलयन के द्वारा ऑक्सीकृत हो जाता है तथा टॉलन अभिकर्मक के साथ रजत दर्पण देता है। (A) अपचयन पर C3H8O (B) देता है। हाइड्रोब्रोमिक अम्ल की क्रिया के पश्चात् मैग्नीशियम के साथ गर्म करने पर (B) एक अन्य यौगिक (C) देता है, जो ऐथिलीन ऑक्साइड के साथ क्रिया करके C5H12O (D) देता है। यौगिक ( D ) ऑक्सीकरण पर C5H10O2 (E) देता है। यौगिक (A) से (E) तक की पहचान कीजिए ।
View SolutionAn organic compound C_(6))H_(12)(X) on reduction gives C_(6)H_(14)(Y) . X on ozonolysis gives two aldehydes C_(2)H_(4)O(I) and C_(4)H_(8)O(II) . Identify the compounds X, Y and aldehydes (I) and (II).
View SolutionAn organic compound A (C2H3N) on reduction with SnCl2/HCl gives B (C2H4O) which reduces Tollen's reagent. Compound B on reduction with N2H4/C2H5ONa gives C (C2H6). Identify the compound A, B and C. Explain the reactions involved.
View SolutionCompound (A) with molecular formula C_(2)H_(4)O reduces Tollen's reagent. (A) on treatment with HCN gives compound (B). Compound (B) on hydrolysis with an acid gives compound ( C) with molecular formula C_(3)H_(6)O which is an optically active compound. Compound (A) on reduction with N_(2)H_(4)//C_(2)H_(5)ONa gives a hydrocarbon (D) of molecular formula C_(2)H_(6) . Identify (A), (B), ( C) and (D) and explain the reactions.
View SolutionAn organic compound A(C_(6)H_(10)) on reduction first gives B(C_(6)H_(12)) and finally C(C_(6)H_(14)) . Compound A on ozonolysis followed by hydrolysis gives two aldehydes D(C_(2)H_(4)O) and E(C_(2)H_(2)O_(2)) . Oxidation of B with acidified KMnO_(4) gives the acid F(C_(4)H_(8)O_(2)) . determine the structures of the compounds A to F with reasoning.
View SolutionAn organic compound on reduction C_(6)H_(12)(X), C_(6)H_(14)(Y) gives. X two aldehydes on ozonation C_(2)H_(4)O (I) and C_(4)H_(8)O (II) gives. Identify the compounds X, Y and aldehyde (I) and (II).
View Solution

