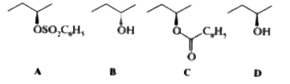
Give stereochemical formulas for A-D. are enantiomers of each of other.

Compound A is a benzenesulfonate ester which forms with retention of configuration from (R)-2-butanol. B is the SN2 product formed by reactions with hydroxide which occurs with inversion of configuratio. C is a benzoate ester. Fomration of which does ot afect the configuration at the chirality centre. Saponification of C to from D does not effect the chirality center either. since it is an acyl substitution reaction.
Topper's Solved these Questions
- CARBOXYLIC ACIDS AND THEIR DERIVATIVESBOOK - MS CHOUHANCHAPTER - CARBOXYLIC ACIDS AND THEIR DERIVATIVESEXERCISE - ADDITIONAL OBJECTIVE QUESTIONS (SINGLE CORRECT CHOICE TYPE)
18 Videos
- CARBOXYLIC ACIDS AND THEIR DERIVATIVESBOOK - MS CHOUHANCHAPTER - CARBOXYLIC ACIDS AND THEIR DERIVATIVESEXERCISE - ADDITIONAL OBJECTIVE QUESTIONS (Linked Comprehension Type Paragraph for Questions)
8 Videos
- CARBOXYLIC ACID AND THEIR DERIVATIVES BOOK - MS CHOUHANCHAPTER - CARBOXYLIC ACID AND THEIR DERIVATIVES EXERCISE - Subjective Problems
1 Video
- CONJUGATED UNSATURATED SYSTEMSBOOK - MS CHOUHANCHAPTER - CONJUGATED UNSATURATED SYSTEMSEXERCISE - Additional Objective Questions (Multiple Correct Choice Type)
4 Videos
Similar Questions
Choose the structure that is an intermediate in the base catalyzed hydrolysis of acetonitrile into acetamide.
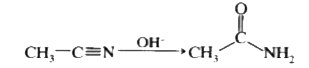
View Solution4 A neutral, resolvable organic compound A has molecular formula (C8H16O2). A on treatment with LiAlH4 gives isomeric B and C(C4H10O) of which only B is optically active. B on treatment with acidified dichromate solution gives D(C4H8O), which on refluxing with dilute NaOH followed by acidification of product gave E(C8H14O). E on heating with N2H4 in alkaline medium affords F(C8H16) which on treatment with B.H/H,O,/HO produced a resolvable G (C8H18O). G on treatment with acidified dichromate solution produced H(C8H16O), which on treatment with MCPBA produces A.
Compound (A) is
View Solution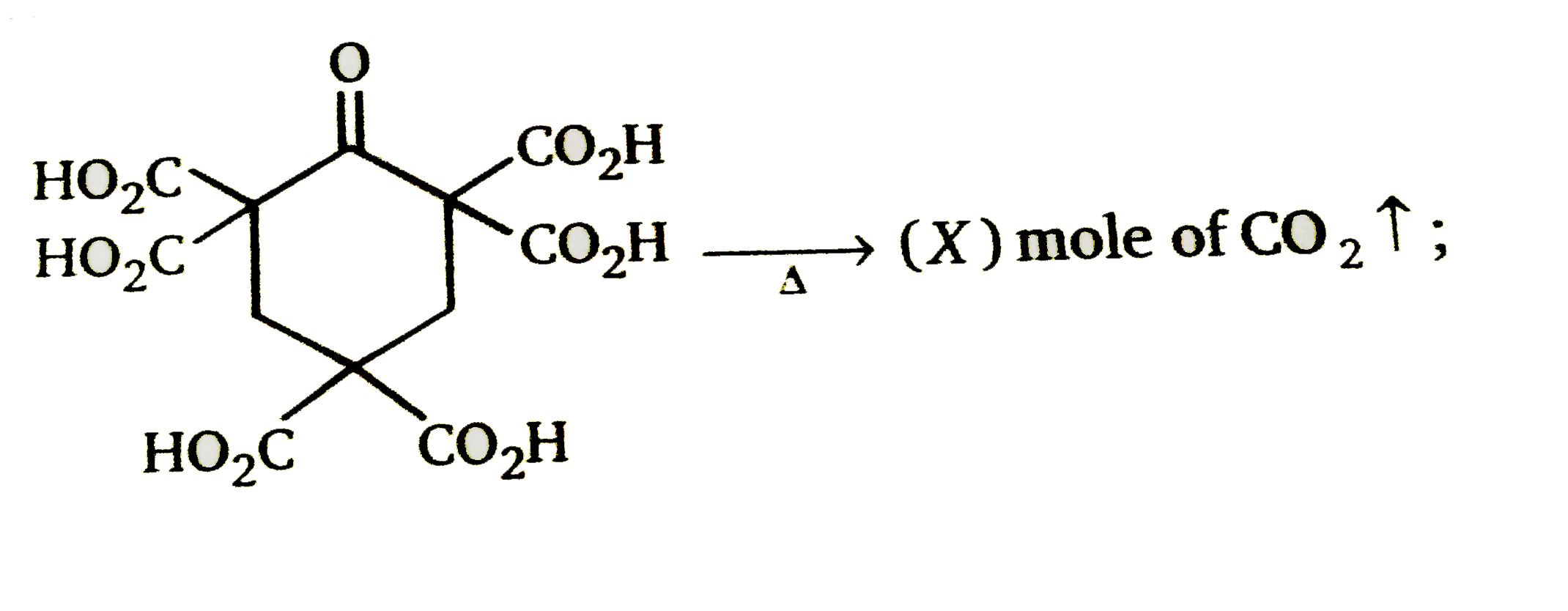
(Y) is including stereoisomers. Value of (X+Y) will be
View SolutionConsider the given reaction and identify the product P
CH3−CH=CH−CH2−OHHBr−−→SN1P
View SolutionWrite the appropriate structural formule for:
a. A cyclic molecule that is a consitutional isomer of cyclohexane
b. Molecules with the formula C6H12 that contain one ring and are enantiomers of each other.
c. Molecules with formula C6H12 that contian one ring and are diastereomers of each other.
d. Molecules with formula C6H12 that contain no ring and are enantiomers of each other.
e. Molecules with formula C6H12 that contain no ring and are diastereomers of each other.
View SolutionGive the stereochemical products of the following :
 .
.
View SolutionGive the stereochemical products of the following :
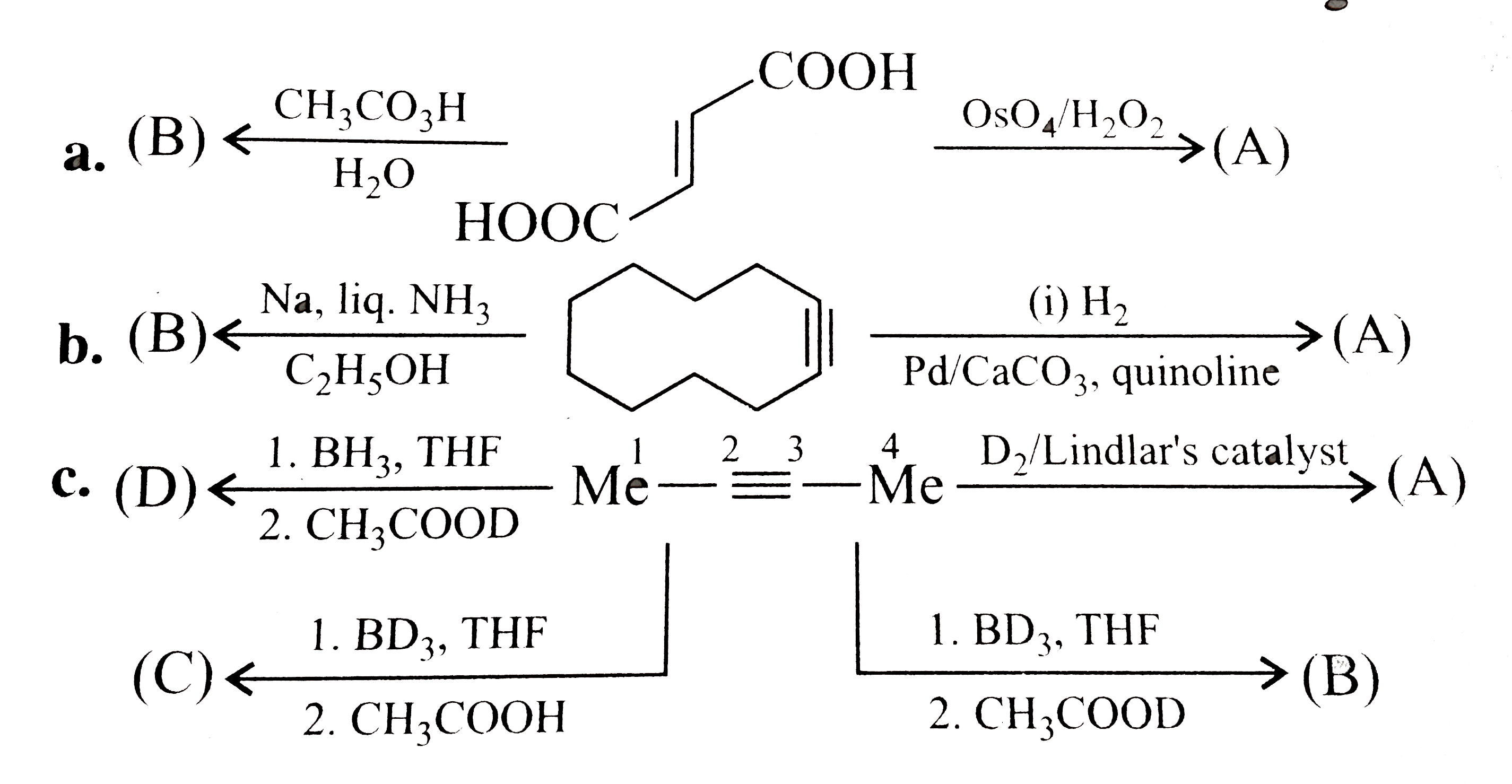
View SolutionGive the stereochemical forculae for (A),(B)(C), and (D) and also give the reactions involved.
View Solutionthe correct stereochemical formula of trans- 3 -chloro- 1 -phenylbut- 1 -ene is
View SolutionEnantiomers are compounds that are__of each other
View SolutionThe two projection formulae that represent a pair of enantiomers are.
View SolutionDraw the enantiomers of 3-bromo-cyclohexene and give R/S designation for each.
View SolutionWhat are enantiomers? Give example.
View SolutionThe stereoisomers which are superimposable mirror images of each other are called enantiomers.
View SolutionWrite the appropriate structural formule for: a. A cyclic molecule that is a consitutional isomer of cyclohexane b. Molecules with the formula C_(6)H_(12) that contain one ring and are enantiomers of each other. c. Molecules with formula C_(6)H_(12) that contian one ring and are diastereomers of each other. d. Molecules with formula C_(6)H_(12) that contain no ring and are enantiomers of each other. e. Molecules with formula C_(6)H_(12) that contain no ring and are diastereomers of each other.
View Solution

