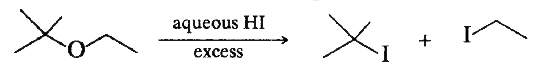
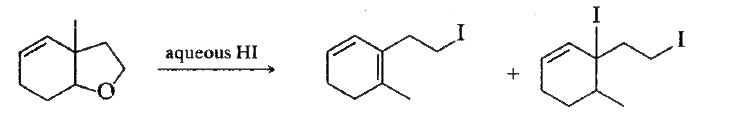
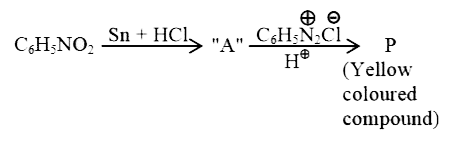
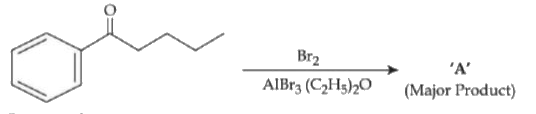Consider the reaction of 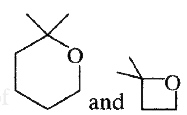 with aqueous HI.
with aqueous HI.
Give the mechanism and product of the faster reaction
Text Solution
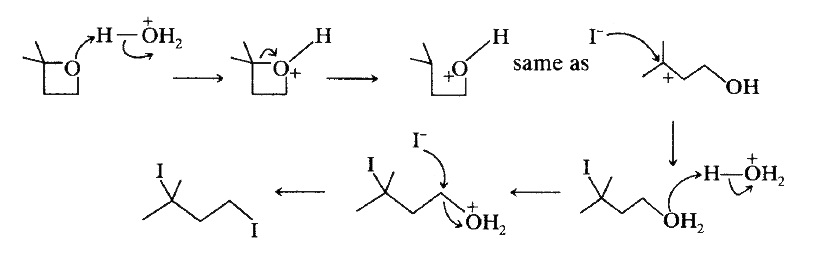
Several variations of this mechanism are acceptable, as long as they make chemical sense and lead to the same diiodide.
|
Topper's Solved these Questions
- ALCOHOLS AND ETHERSBook:MS CHOUHANChapter:ALCOHOLS AND ETHERSExercise:ADDITIONAL OBJECTIVE QUESTIONS (SINGLE CORRECT CHOICE TYPE)
Explore 26 Videos
- ALCOHOLS AND ETHERSBook:MS CHOUHANChapter:ALCOHOLS AND ETHERSExercise:ADDITIONAL OBJECTIVE QUESTIONS (Linked Comprehension Type ) Paragraph for Question 1 to 4
Explore 1 Video
- ALCOHOL, ETHERS AND EPOXIDESBook:MS CHOUHANChapter:ALCOHOL, ETHERS AND EPOXIDESExercise:Subjective Problems
Explore 1 Video
- ALCOHOLS FROM CARBONYL COMPOUNDSBook:MS CHOUHANChapter:ALCOHOLS FROM CARBONYL COMPOUNDSExercise:Additional Objective Questions (Multiple Correct Choice Type )
Explore 1 Video
Similar Questions
Knowledge Check
Consider the reaction

The end product of the reaction isA B
B C
C D
D
Propyne on reaction with aqueous chlorine gives-
A1,1,2,2−TetrachloropropaneB1,2−DichloropropeneC1,1−DichloropropanoneD2,2−DichloropropanoneConsider the reaction.
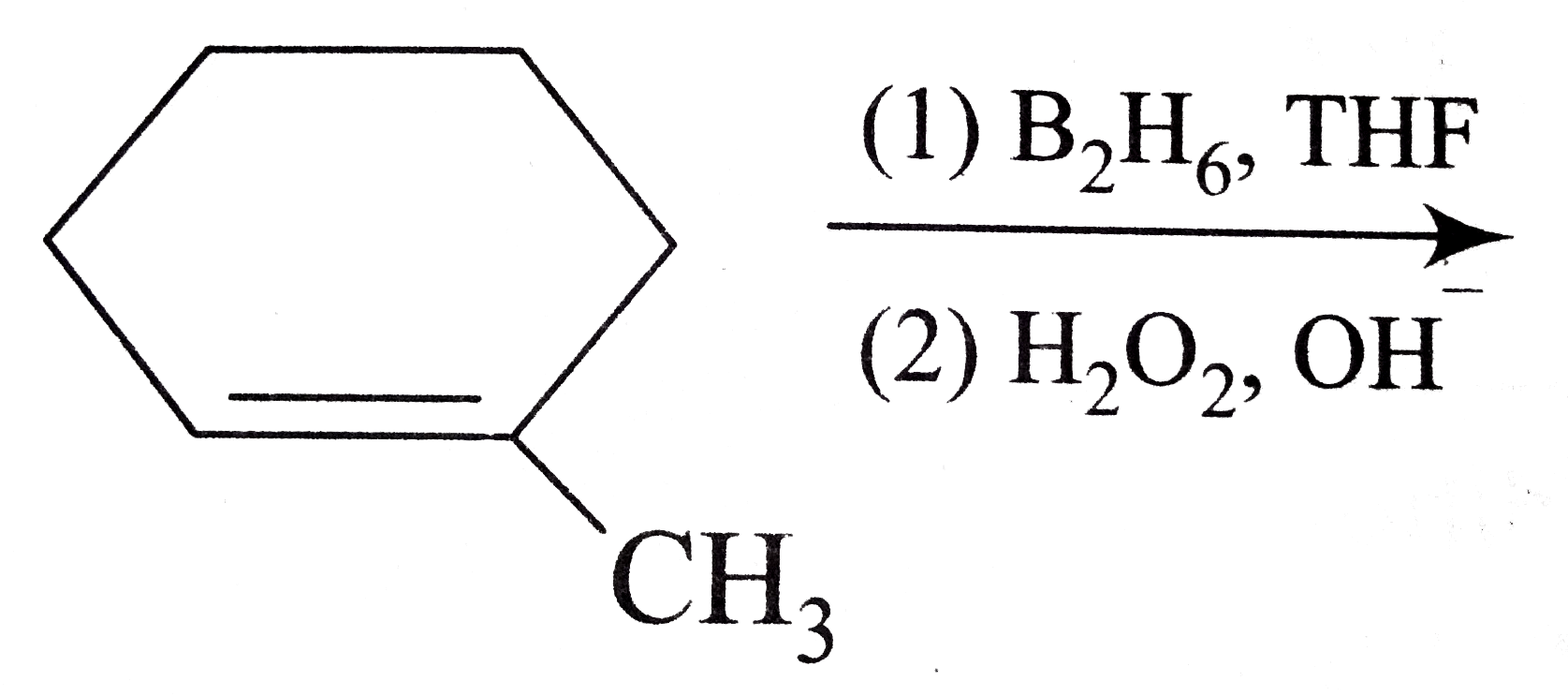
The product formed isA(±)cis−2−MethylcyclohexanolB(±)−trans−2-MethylcyclohexanolC2-MethylcyclohexanolD1-Methylcyclohexanol


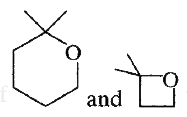 with aqueous HI.
with aqueous HI.