The diagram represents an isotope of hydrogen (H). Answer the following:
If an isotope of 'H' has mass no. = 3, how many neutrons does it have.
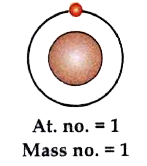
Step by step video solution for The diagram represents an isotope of hydrogen (H). Answer the following: If an isotope of 'H' has mass no. = 3, how many neutrons does it have. by Chemistry experts to help you in doubts & scoring excellent marks in Class 8 exams.
Updated on:21/07/2023Similar Questions
The diagram represents an isotope of hydrogen (H). Answer the following: Are isotopes atoms of the same element or different elements.
View SolutionThe diagram represents an isotope of hydrogen (H). Answer the following: Do isotopes have the same atomic number or the same mass number.
View SolutionThe diagram represents an isotope of hydrogen (H). Answer the following:
If an isotope of 'H' has mass no. = 2, how many electrons does it have.
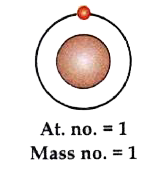
View SolutionThe diagram represents an isotope of hydrogen (H). Answer the following: Which sub-atomic particles in the 3 isotopes of 'H' are the same.
View SolutionWhich isotope of hydrogen has no neutron?
View Solutionएक परमाणु जिसकी परमाणु संख्या 8 है, उसके तीन समस्थानिकों का द्रव्यमान 16, 17 और 18 हैं। इन तीनों समस्थानिकों के नाभिक में कितने प्रोटॉन तथा न्यूट्रॉन होंगे?
View SolutionAnswer in one word. How many isotopes of hydrogen are there?
View Solution