The wrong statement in the case of an ideal gas is Solution in Telugu
It cannot be converted into a liquid
There are no attractive forces between molecules
All molecules of the gas move with the same speed
At a given temperature PV is proportional to the amount of the gas
The correct Answer is:C
Step by step video & text solution for The wrong statement in the case of an ideal gas is by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- STATES OF MATTERBook:AAKASH SERIESChapter:STATES OF MATTERExercise:OBJECTIVE EXERCISE -2
Explore 140 Videos
- STATES OF MATTERBook:AAKASH SERIESChapter:STATES OF MATTERExercise:OBJECTIVE EXERCISE -3
Explore 19 Videos
- STATES OF MATTERBook:AAKASH SERIESChapter:STATES OF MATTERExercise:SUBJECTIVE EXERCISE -3 (VERY SHORT ANSWER QUESTIONS)
Explore 18 Videos
- SATURATED HYDROCARBONSBook:AAKASH SERIESChapter:SATURATED HYDROCARBONSExercise:QUESTIONS FOR DESCRIPTIVE ANSWERS
Explore 10 Videos
- STOICHIOMETRYBook:AAKASH SERIESChapter:STOICHIOMETRYExercise:QUESTIONS FOR DESCRIPTIVE ANSWERS
Explore 59 Videos
Similar Questions
A gas of volume 2000ml is kept in a vessel at a pressure of 103 pascals at a temperature of 27∘C. If the pressure is increased to 105 pascals at the same temperature, the volume of the gas becomes
View SolutionVan der Waal's real gas, acts as an ideal gas, at which conditions
View SolutionWhat are dispersion forces ?
View SolutionWhy boiling and melting points of hydrocarbons are low ?
View SolutionDistinguish between element and compound?
View SolutionPressure versus temperature graphs of an ideal gas are as shown in figure. Choose the wrong statement
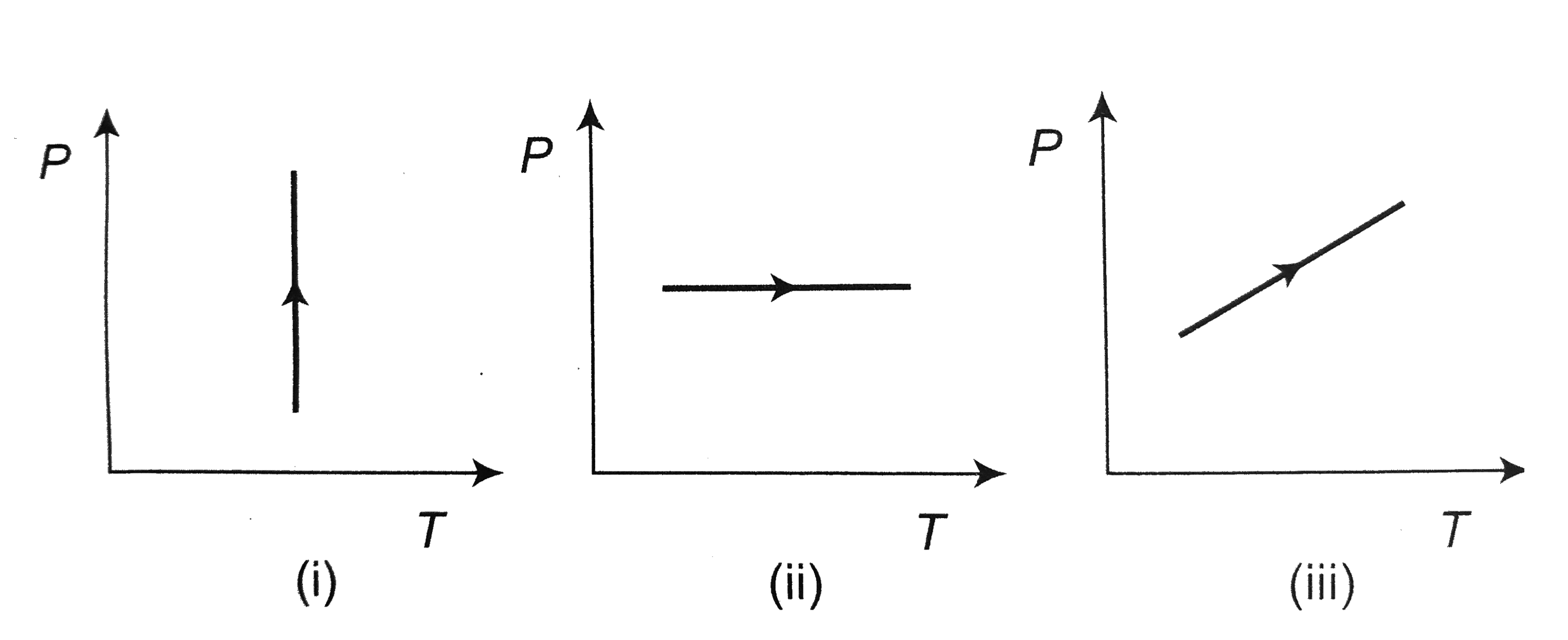
View SolutionFor isothermal expansion in case of an ideal gas :
View SolutionWhich is not true in case of an ideal gas?
View SolutionIn an isothermal expansion of an ideal gas. Select wrong statement:
View SolutionFor isothemal expansion in case of an ideal gas:
View SolutionWhich is not true in case of an ideal gas?
View SolutionPressure versus temperature graph of an ideal gas are as shown in figure-2.39. Choose the wrong statement
View SolutionP-T graphs of an ideal gas are as shown in figures-2.42 below. Choose the wrong statement from the options given:
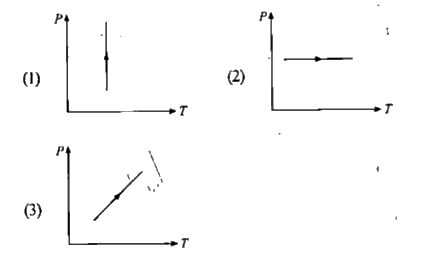
View SolutionWhich one of the following statements is wrong in case Bhopal gas tragedy ?
View SolutionFor isothermal expansion in case of an ideal gas :
View Solution
