Which one of the following is a strong acid?
CH3COOH
HCl
H2C2O4
H2CO3
The correct Answer is:B
To determine which one of the following is a strong acid among the options provided (acetic acid, hydrochloric acid, oxalic acid, and carbonic acid), we can follow these steps:
Step 1: Understanding Strong Acids
Strong acids are substances that completely dissociate into their ions in an aqueous solution. This means that they release a high concentration of hydrogen ions (H⁺) when dissolved in water.
Step 2: Analyzing the Given Options
We have four acids to consider:
1. Acetic Acid (CH₃COOH) - A weak acid that partially dissociates in solution.
2. Hydrochloric Acid (HCl) - A strong acid that completely dissociates in solution.
3. Oxalic Acid (C₂H₂O₄) - A weak acid that partially dissociates in solution.
4. Carbonic Acid (H₂CO₃) - A weak acid that partially dissociates in solution.
Step 3: Comparing the Acids
- Acetic Acid: It has a conjugate base (CH₃COO⁻) that is relatively stable but does not dissociate completely, making it a weak acid.
- Hydrochloric Acid: It has a conjugate base (Cl⁻) that is highly stable due to its high electronegativity and complete dissociation, making it a strong acid.
- Oxalic Acid: Its conjugate base (HC₂O₄⁻) is less stable, indicating that it does not dissociate completely, thus it is a weak acid.
- Carbonic Acid: Its conjugate base (HCO₃⁻) is also less stable, indicating partial dissociation, making it a weak acid.
Step 4: Conclusion
Among the four acids, Hydrochloric Acid (HCl) is the only strong acid as it completely dissociates in solution and has the most stable conjugate base (Cl⁻).
Final Answer
The strong acid among the options is Hydrochloric Acid (HCl).
---
Strong acids are substances that completely dissociate into their ions in an aqueous solution. This means that they release a high concentration of hydrogen ions (H⁺) when dissolved in water.
We have four acids to consider:
1. Acetic Acid (CH₃COOH) - A weak acid that partially dissociates in solution.
2. Hydrochloric Acid (HCl) - A strong acid that completely dissociates in solution.
3. Oxalic Acid (C₂H₂O₄) - A weak acid that partially dissociates in solution.
4. Carbonic Acid (H₂CO₃) - A weak acid that partially dissociates in solution.
- Acetic Acid: It has a conjugate base (CH₃COO⁻) that is relatively stable but does not dissociate completely, making it a weak acid.
- Hydrochloric Acid: It has a conjugate base (Cl⁻) that is highly stable due to its high electronegativity and complete dissociation, making it a strong acid.
- Oxalic Acid: Its conjugate base (HC₂O₄⁻) is less stable, indicating that it does not dissociate completely, thus it is a weak acid.
- Carbonic Acid: Its conjugate base (HCO₃⁻) is also less stable, indicating partial dissociation, making it a weak acid.
Among the four acids, Hydrochloric Acid (HCl) is the only strong acid as it completely dissociates in solution and has the most stable conjugate base (Cl⁻).
The strong acid among the options is Hydrochloric Acid (HCl).
Topper's Solved these Questions
- STUDY OF ACIDS, BASES AND SALTSBook:ICSEChapter:STUDY OF ACIDS, BASES AND SALTSExercise:QUESTIONS FOR PRACTICE ON EXAMINATION PATTERN (SECTION II)
Explore 13 Videos
- STUDY OF ACIDS, BASES AND SALTSBook:ICSEChapter:STUDY OF ACIDS, BASES AND SALTSExercise:WORKSHEET-1
Explore 18 Videos
- STUDY OF ACIDS, BASES AND SALTSBook:ICSEChapter:STUDY OF ACIDS, BASES AND SALTSExercise:ILLUSTRATIVE ASSIGNMENTS
Explore 19 Videos
- SPECIMEN QUESTION PAPER (CHEMISTRY)Book:ICSEChapter:SPECIMEN QUESTION PAPER (CHEMISTRY)Exercise:Questions
Explore 40 Videos
- STUDY OF COMPOUNDS - AMMONIA Book:ICSEChapter:STUDY OF COMPOUNDS - AMMONIA Exercise:UNIT TEST PAPER 7B - AMMONIA
Explore 22 Videos
Similar Questions
Knowledge Check
Which one of the following is the strongest acid ?
ACH3COOHBCCl3COOHCCHCl2COOHDCH2ClCOOHWhich one of the following is the strongest acid?
A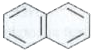 B
B C
C D
D
Which one of the following is most acidic ?
View SolutionWhich one of the following is the most acidic?
View SolutionWhich one of the following is used as an acidic flux in metallurgy ?
View SolutionWhich one of the following salt is most acidic in water?
View SolutionEach 10−3M of following four acids are taken. Their PH values are given in brackets. Which of the following is relatively strong acid?
View SolutionWhich of the following is a strong electrolyte ?
View Solution