Read the following statements:
(I) Atomic size of following pair are almost same
Fe, Co, Al,Ga , Zr, Hf
(II) E.A. order of Li, Be,B,C,N,O is
Be<N<B<Li<C<O
(III) Ionisation energy of
Al<Ga&Zr<Hf
(IV) Ionic radius
O−2<F−<Na+<Mg+2
Select the correct statements(s)
I, III, IV
I, II, III
II, III, IV
All the four
The correct Answer is:B
টপার এই প্রশ্নগুলো সমাধান করেছে
- INORGANIC CHEMISTRYBOOK - ALLENCHAPTER - INORGANIC CHEMISTRYEXERCISE - PERIODIC TABLE (MATCH THE COLUMN TYPE QUESTIONS)
5 Videos
- INORGANIC CHEMISTRYBOOK - ALLENCHAPTER - INORGANIC CHEMISTRYEXERCISE - CHEMICAL BONDING
120 Videos
- HYDROGENBOOK - ALLENCHAPTER - HYDROGENEXERCISE - EXERCISE-5
18 Videos
- Ionic EquilibriumBOOK - ALLENCHAPTER - Ionic EquilibriumEXERCISE - All Questions
26 Videos
সম্পর্কিত প্রশ্ন
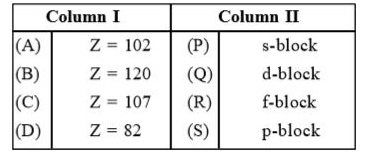
View SolutionWhich of the following is/are correct :-
View SolutionAssertion : Cu react with HCl & form H_(2) gas
Reason : Cu above then hydrogen in electro chemical series
View SolutionThe percentage degree of hydrolysis of a salt of weak acid (HA) and weak base (BOH) in its 0.1 M solution is found to be 10%. If the molarity of the solution is 0.05 M, the percentage hydrolysis of the salt should be :
View SolutionCorrect order of IE1 are:
(P) Li<B<Be<C (Q) O<N<F
(R) Be<N<Ne
View SolutionFind the total number of correct orders among the following. (a) Order of electron affinity : S gt O^(-) (b) Order of ionic radius: AI^(3+) lt Mg^(2+) lt O^(2-) (c) Order of electronegativity: Si lt P lt C lt N (d) Order of atomic radius: O lt C lt S lt Se (e) Order of second ionization energy: C lt N lt F lt O (f) Order of ionic radii : S^(2-) lt CI^(-) lt K^(+) lt Ca^(2+)
View SolutionWith reference to 1^(st) IP which are correct. (a) Li lt C (b) O lt N (c ) Be lt N lt Ne
View Solutionआयनिक त्रिज्या के बढ़ते क्रम में व्यवस्थित कीजिए । N3−,O2−,F−,Na+,Mg2+ एवं Al3+
View SolutionExplain the following data : Ionisation energy Cl lt H lt O lt N lt F .
View SolutionAmong the second period elements the actual ionisation enthalpies are in the order Li<B<Be<C<O<N<F<Ne . Explain why?
Be has higher (ΔiH)1 than B
View SolutionAmong the second period elements the actual ionisation enthalpies are in the order Li<B<Be<C<O<N<F<Ne . Explain why?
O has lower (ΔiH)1 than N and F ?
View Solutionआयनिक त्रिज्याओं के कौनसे क्रम सही हैं :
(a)Li<Be<na , (b)Ni<Cu<Zn , (c)Ti<V<Cr , (d)Ti<Zr≅Hf
सही उत्तर है
View SolutionIP का सही क्रम है:-
(a)Li<B<Be<C
(b)O<N<F
(c) Be<N<Ne
View SolutionCorrect orders of IE1 are
(i) Li<B<Be<C
(ii) O<N<F
(iii) Be<N<Ne
View SolutionWhich of the following is/are the correct order of ionic mobility ?
(1) Li+<Na+<K+
(2) Na+<Mg2+<Al3+
(3) Al3+<Mg2+<Na2+
(4) Li+<Na+<K+
View Solution

