Which of the following pairs of species would you expect to have largest difference in spin only magnetic moment ?
A
O2,O+2
B
O2O2−2
C
O+2,O2−2
D
O−2,O+2
Text Solution
The correct Answer is:B
SpeciesNo. of unpairedO22O+21O−21O−220
|
Topper's Solved these Questions
- INORGANIC CHEMISTRYBook:ALLENChapter:INORGANIC CHEMISTRYExercise:COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS
Explore 113 Videos
- INORGANIC CHEMISTRYBook:ALLENChapter:INORGANIC CHEMISTRYExercise:p-BLOCK ELEMENT
Explore 80 Videos
- INORGANIC CHEMISTRYBook:ALLENChapter:INORGANIC CHEMISTRYExercise:PERIODIC TABLE (MATCH THE COLUMN TYPE QUESTIONS)
Explore 5 Videos
- HYDROGENBook:ALLENChapter:HYDROGENExercise:EXERCISE-5
Explore 18 Videos
- Ionic EquilibriumBook:ALLENChapter:Ionic EquilibriumExercise:All Questions
Explore 26 Videos
Similar Questions
Knowledge Check
Which of the following compounds would you expect to have a dipole moment ?
A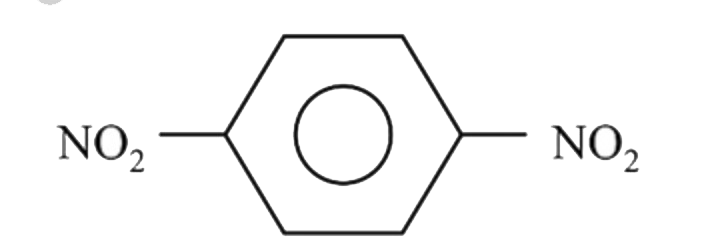 B
B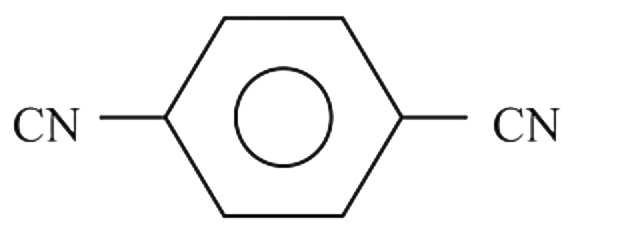 C
C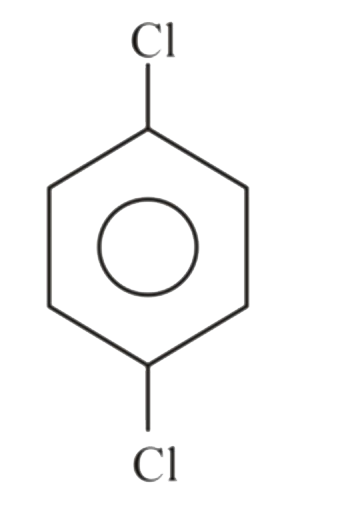 D
D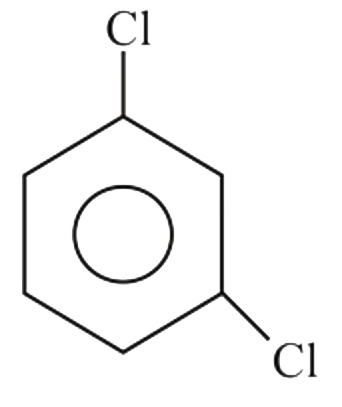
Which of the following would be expected to have the largest antropy per mole
ASO2Cl2(s)BSO2Cl2(g)CSO2Cl2(l)DSO2(g)The complex showing a spin - only magnetic moment
A[Ni(CO)4]B[NiCl4]2−C[Ni(PPh3)4]D[Ni(CN)4]2−
Which of the following species have same magnetic moment?
View SolutionThe complex having the highest spin-only magnetic moment is
View SolutionWhich of the following is expected to have lowest magnetic moment-
View SolutionWhich of the following is expected to have highest magnetic moment?
View SolutionWhich of the following species have same magnetic moment?
View Solution

