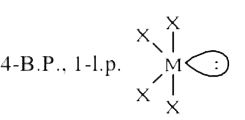Shape of molecule having 4-bond pair and one lone pair is :-
Trigonal bipyramidal
T-Shape
See saw
Square planar
The correct Answer is:C
Topper's Solved these Questions
- INORGANIC CHEMISTRYBook:ALLENChapter:INORGANIC CHEMISTRYExercise:COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS
Explore 113 Videos
- INORGANIC CHEMISTRYBook:ALLENChapter:INORGANIC CHEMISTRYExercise:p-BLOCK ELEMENT
Explore 80 Videos
- INORGANIC CHEMISTRYBook:ALLENChapter:INORGANIC CHEMISTRYExercise:PERIODIC TABLE (MATCH THE COLUMN TYPE QUESTIONS)
Explore 5 Videos
- HYDROGENBook:ALLENChapter:HYDROGENExercise:EXERCISE-5
Explore 18 Videos
- Ionic EquilibriumBook:ALLENChapter:Ionic EquilibriumExercise:All Questions
Explore 26 Videos
Similar Questions
Knowledge Check
Shape of a molecule having four bond pairs and two lone pairs of electrons will be
Asquare plannarBTetrahedralCLinearDoctahedralA molecule having 3 bond pairs and 2 lone pairs will have ?
AT - shape geometryBTrigonal planar geometryCLinear geometryDSquare pyramidal geometryThe shape of a molecule which has 3 bond paires and one lone pair is :
AoctahedralBpyramidalCtriangular planarDtetrahedral
Valence shell electron pair repusin theory (VSEPR) can be used
to perdict the approximate shape of molecules. Electrons in
bonds and in lone pairs can ve thought of as charge cloud that
repel one another and stay as far apart possible, thus causing molecules to assume specific shapes.
The repulsive interacions of electro paris decrease in the order :
Lone pair-lone pair > Lone pair -bond pair >Bond pair -bond
pair.
These replusions effect result in deviations from
idealized shapes and alternative in bond angles in molecules.
The species which has pyramidal shape is :
View SolutionValence shell electron pair repulsion theory (VSEPR) can be used to predict the approximate shape of molecules. Electrons in bonds and in lone pairs can be thought of as charge cloud that repel one another stay as far apart possible, thus causing molecules to assume specific shapes.
The repulsive interactions of electro pair is decrease in the order :
Lone pair-lone pair > Lone pair -bond pair > Bond pair -bond pair.
These repulsions effect result in deviations from idealized shapes and alteration in bond angles in molecules
Which of the following is linear ?
View SolutionHybridisation And Shapes Of Molecules Containing Bond Pairs And Lone Pairs Of Electrons
View SolutionValence shell electron pair repusin theory (VSEPR) can be used to perdict the approximate shape of molecules. Electrons in bonds and in lone pairs can ve thought of as charge cloud that repel one another and stay as far apart possible, thus causing molecules to assume specific shapes.
The repulsive interacions of electro paris decrease in the order :
Lone pair-lone pair > Lone pair -bond pair > Bond pair -bond pair.
These replusions effect result in deviations from idealized shapes and alternative in bond aegles in molecules.
The species which has pyramidal shape is :
View SolutionValence shell electron pair repusin theory (VSEPR) can be used to perdict the approximate shape of molecules. Electrons in bonds and in lone pairs can ve thought of as charge cloud that repel one another and stay as far apart possible, thus causing molecules to assume specific shapes.
The repulsive interacions of electro paris decrease in the order :
Lone pair-lone pair > Lone pair -bond pair > Bond pair -bond pair.
These repulsions effect result in deviations from idealized shapes and ateration in bond angles in molecules
Which of the following is linear ?
View Solution