Which of the following reaction is nonspontaneous :-
2F2+2H2O→4HF(aq)+O2
Cl2+H−OH→HCl+HOCl
Br2+H−OH→HBr+HOBr
2I2+2H2O→4HI+O2
The correct Answer is:D
I2+H2O→HI+O2 reaction non spontaneous because I2 is strong reducing agent
Topper's Solved these Questions
- INORGANIC CHEMISTRYBOOK - ALLENCHAPTER - INORGANIC CHEMISTRYEXERCISE - s-BLOCK ELEMENTS
21 Videos
- INORGANIC CHEMISTRYBOOK - ALLENCHAPTER - INORGANIC CHEMISTRYEXERCISE - METALLURGY
21 Videos
- INORGANIC CHEMISTRYBOOK - ALLENCHAPTER - INORGANIC CHEMISTRYEXERCISE - COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS
113 Videos
- HYDROGENBOOK - ALLENCHAPTER - HYDROGENEXERCISE - EXERCISE-5
18 Videos
- Ionic EquilibriumBOOK - ALLENCHAPTER - Ionic EquilibriumEXERCISE - All Questions
26 Videos
Similar Questions
Which of the following order of L.E. is not correct :-
View SolutionIn order to refine "copper" it is melted in a furnace and it is stirred with green logs of wood. The purpose is :-
View SolutionThe oxidation state of Mo in its oxido-complex species [Mo2O4(C2H4)(H2O)2]2− is
View SolutionAssertion : Cu react with HCl & form H_(2) gas
Reason : Cu above then hydrogen in electro chemical series
View SolutionThe percentage degree of hydrolysis of a salt of weak acid (HA) and weak base (BOH) in its 0.1 M solution is found to be 10%. If the molarity of the solution is 0.05 M, the percentage hydrolysis of the salt should be :
View SolutionWhich of the following reactions is not a redox reaction?
View SolutionConsider the following reactions : Which of the following are stereospecific reactions ?
View SolutionConsider the following reactions :
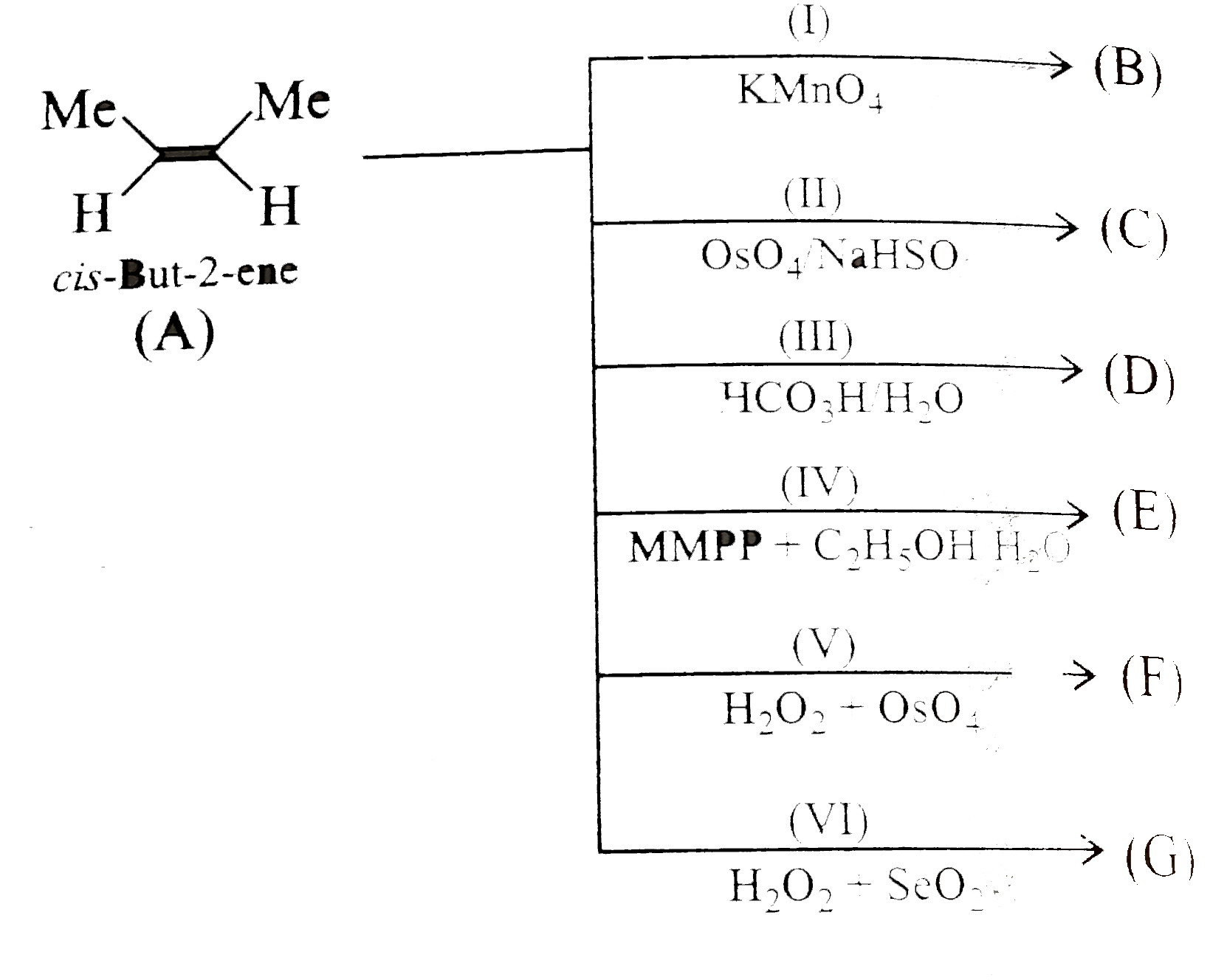
Which of the following regioselective reactions ?
View SolutionFor a certain reaction ΔH=−50kJ and ΔS=−80jK−1, at what temperature does the reaction turn from spontaneous to nonspontaneous?
View SolutionStatement-1 : An exothermic reaction, nonspontaneous at high temperature, may become spontaneous at low temperature. Statement-2 : Entropy of an exothermic reaction always decrease with decrease in temperature.
View Solutionनिम्न अभिक्रियाओं में कौन सी अभिक्रिया ऊष्माशोषी हैं :-
View SolutionFor a certain reaction, ΔH∘ =- 219 kJ and ΔS∘ = - 21JK−1 Determine whether the reaction is spontaneous or nonspontaneous.
View Solutionनिम्न रासायनिक अभिक्रियाओं में कौन सी योगात्मक अभिक्रिया है?
View SolutionA certain reaction is nonspontaneous at 298 K. The entropy change during the reaction is 121 J/K . Is the reaction endothermic or exothermic ? What is the minimum value of ΔH for the reaction ?
View SolutionWhich of the following reactions is not a redox reaction?
View Solution

