Differentiate between the following:
Oxidising electrode and reducing electrode.
Step-by-Step Solution
1. Definition of Oxidizing Electrode:
- An oxidizing electrode is the electrode where oxidation occurs. In an electrochemical cell, this is typically the anode. At the anode, a species loses electrons, which is the definition of oxidation.
2. Definition of Reducing Electrode:
- A reducing electrode is the electrode where reduction occurs. In an electrochemical cell, this is typically the cathode. At the cathode, a species gains electrons, which is the definition of reduction.
3. Electrochemical Cell Representation:
- In an electrochemical cell, the anode is the positive electrode and the cathode is the negative electrode. This is crucial for understanding the flow of electrons and ions in the cell.
4. Ion Movement:
- During electrolysis, negative ions (anions) move towards the positive electrode (anode), while positive ions (cations) move towards the negative electrode (cathode). For example, in a KCl solution, Cl⁻ ions move towards the anode and K⁺ ions move towards the cathode.
5. Reactions at Each Electrode:
- At the anode (oxidizing electrode), the oxidation reaction occurs. For instance, Cl⁻ ions lose electrons to form chlorine gas (Cl₂). The half-reaction can be represented as:
2Cl−→Cl2+2e−
- At the cathode (reducing electrode), the reduction reaction occurs. For example, K⁺ ions gain electrons to form solid potassium (K). The half-reaction can be represented as:
K++e−→K(s)
6. Conclusion:
- Therefore, the oxidizing electrode is where oxidation occurs (anode), and the reducing electrode is where reduction occurs (cathode). The anode is associated with the loss of electrons, while the cathode is associated with the gain of electrons.
- An oxidizing electrode is the electrode where oxidation occurs. In an electrochemical cell, this is typically the anode. At the anode, a species loses electrons, which is the definition of oxidation.
- A reducing electrode is the electrode where reduction occurs. In an electrochemical cell, this is typically the cathode. At the cathode, a species gains electrons, which is the definition of reduction.
- In an electrochemical cell, the anode is the positive electrode and the cathode is the negative electrode. This is crucial for understanding the flow of electrons and ions in the cell.
- During electrolysis, negative ions (anions) move towards the positive electrode (anode), while positive ions (cations) move towards the negative electrode (cathode). For example, in a KCl solution, Cl⁻ ions move towards the anode and K⁺ ions move towards the cathode.
- At the anode (oxidizing electrode), the oxidation reaction occurs. For instance, Cl⁻ ions lose electrons to form chlorine gas (Cl₂). The half-reaction can be represented as:
2Cl−→Cl2+2e−
- At the cathode (reducing electrode), the reduction reaction occurs. For example, K⁺ ions gain electrons to form solid potassium (K). The half-reaction can be represented as:
K++e−→K(s)
- Therefore, the oxidizing electrode is where oxidation occurs (anode), and the reducing electrode is where reduction occurs (cathode). The anode is associated with the loss of electrons, while the cathode is associated with the gain of electrons.
Topper's Solved these Questions
Similar Questions
Differentiate between the following:
Seed and fruit
View SolutionDifferentiate between the following:
Stamen and carpel
View SolutionDifferentiate between the following acids :
(a)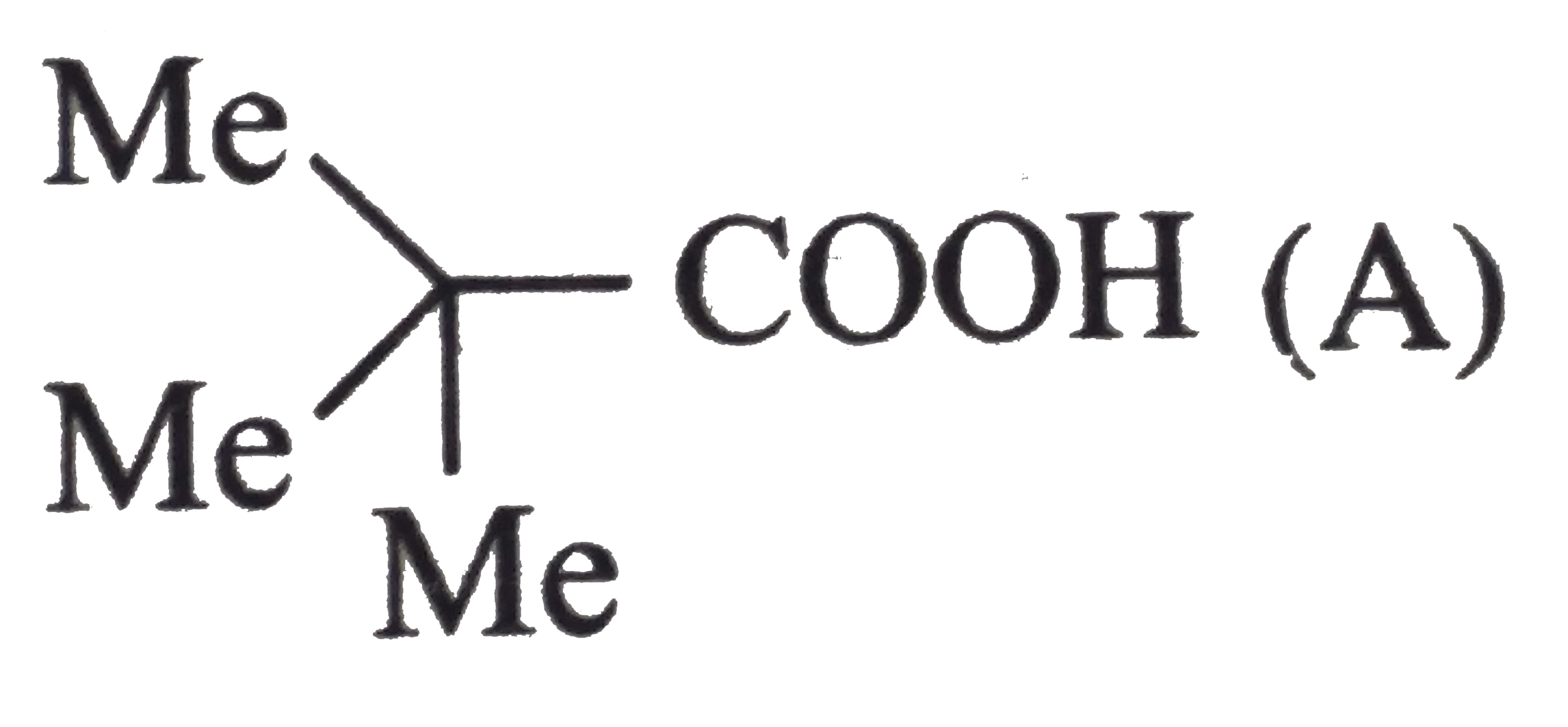
(b)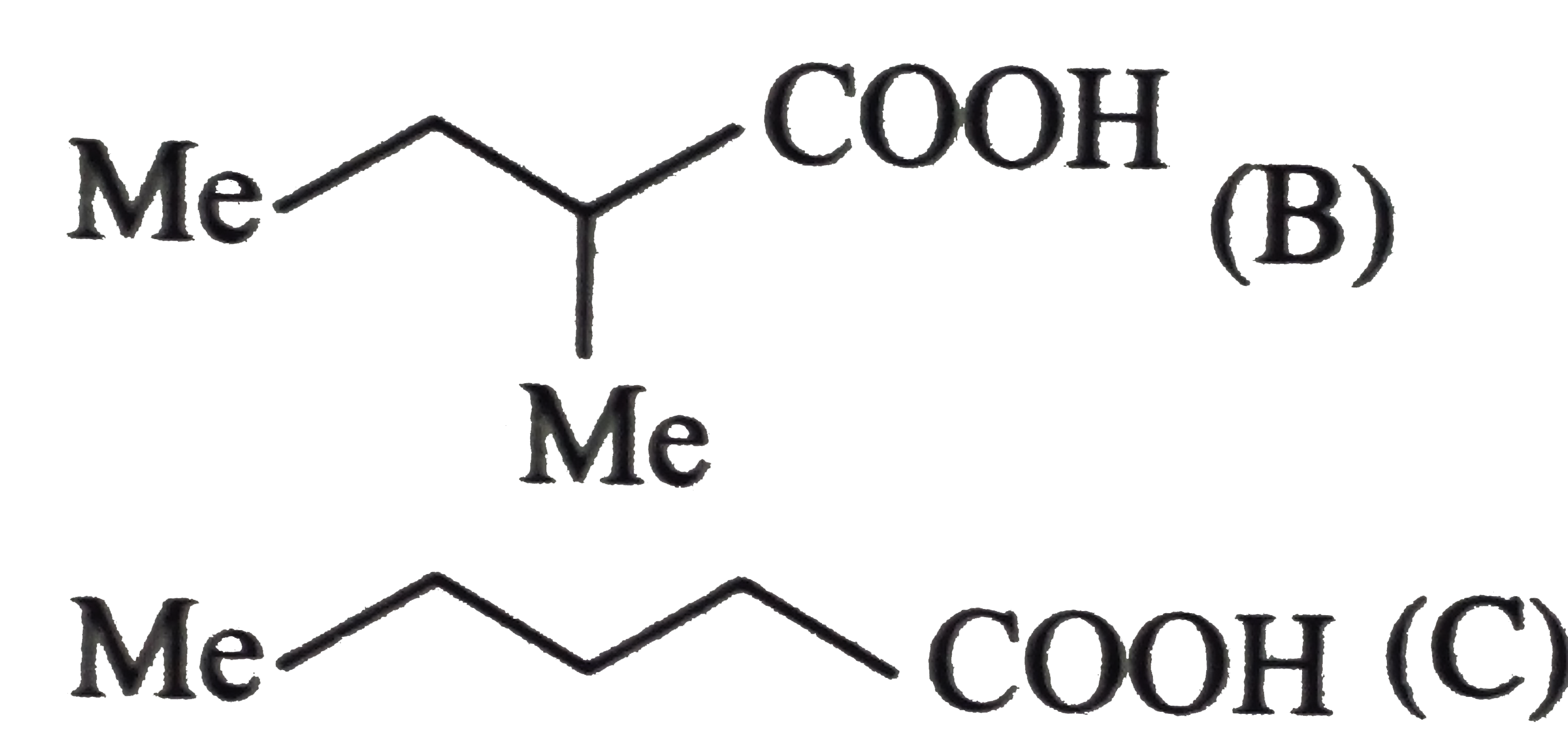
View SolutionDifferentiate between the following
Fibres and Sclereids
View SolutionDifferentiate between the following:
Calyx and corolla
View SolutionDifferentiate between the following:
Lotus and Hydrilla
View SolutionDifferentiate between the following:
Pepsin and Trypsin
View SolutionDifferentiate between the following:
Gigantism and Acromegaly.
View SolutionDifferentiate between the following
Phellem and phelloderm
View SolutionDifferentiate between the following
Trancheid and Vessel
View Solution