For each of the salt : A, B, C and D, suggest a suitable method of its preparation :
C is a soluble salt of copper
To prepare the soluble salt of copper, denoted as C, we can follow these steps:
1. Identify the Soluble Salt of Copper:
- The soluble salt of copper we will focus on is Copper(II) chloride (CuCl2).
2. Choose the Reactants:
- We can prepare CuCl2 by reacting copper carbonate (CuCO3) with hydrochloric acid (HCl).
3. Write the Chemical Reaction:
- The reaction can be represented as:
CuCO3(s)+2HCl(aq)→CuCl2(aq)+H2CO3(aq)
- Here, copper carbonate reacts with hydrochloric acid to produce copper(II) chloride and carbonic acid.
4. Decompose the Product:
- The carbonic acid (H2CO3) formed is unstable and decomposes into water (H2O) and carbon dioxide (CO2), which can be released as gas:
H2CO3(aq)→H2O(l)+CO2(g)
5. Final Product:
- The final product is a solution of copper(II) chloride (CuCl2), which is the soluble salt of copper we aimed to prepare.
Summary of Preparation:
- To prepare the soluble salt of copper (CuCl2), react copper carbonate with hydrochloric acid, leading to the formation of copper(II) chloride and the release of carbon dioxide gas.
- The soluble salt of copper we will focus on is Copper(II) chloride (CuCl2).
- We can prepare CuCl2 by reacting copper carbonate (CuCO3) with hydrochloric acid (HCl).
- The reaction can be represented as:
CuCO3(s)+2HCl(aq)→CuCl2(aq)+H2CO3(aq)
- Here, copper carbonate reacts with hydrochloric acid to produce copper(II) chloride and carbonic acid.
- The carbonic acid (H2CO3) formed is unstable and decomposes into water (H2O) and carbon dioxide (CO2), which can be released as gas:
H2CO3(aq)→H2O(l)+CO2(g)
- The final product is a solution of copper(II) chloride (CuCl2), which is the soluble salt of copper we aimed to prepare.
- To prepare the soluble salt of copper (CuCl2), react copper carbonate with hydrochloric acid, leading to the formation of copper(II) chloride and the release of carbon dioxide gas.
Topper's Solved these Questions
- STUDY OF ACIDS, BASES AND SALTSBook:ICSEChapter:STUDY OF ACIDS, BASES AND SALTSExercise:Questions from Previous ICSE Board Papers
Explore 91 Videos
- STUDY OF ACIDS, BASES AND SALTSBook:ICSEChapter:STUDY OF ACIDS, BASES AND SALTSExercise:FILL IN THE BLANKS
Explore 24 Videos
- STUDY OF ACIDS, BASES AND SALTSBook:ICSEChapter:STUDY OF ACIDS, BASES AND SALTSExercise:WORKSHEET-5
Explore 19 Videos
- SPECIMEN QUESTION PAPER (CHEMISTRY)Book:ICSEChapter:SPECIMEN QUESTION PAPER (CHEMISTRY)Exercise:Questions
Explore 40 Videos
- STUDY OF COMPOUNDS - AMMONIA Book:ICSEChapter:STUDY OF COMPOUNDS - AMMONIA Exercise:UNIT TEST PAPER 7B - AMMONIA
Explore 22 Videos
Similar Questions
For each of the salt : A, B, C and D, suggest a suitable method of its preparation :
D is a soluble salt of zinc.
View SolutionFor each of the salt : A, B, C and D, suggest a suitable method of its preparation :
B is an insoluble salt.
View SolutionFor each of the salt : A, B, C and D, suggest a suitable method of its preparation :
A is a sodium salt.
View SolutionMatch the salts in List - 1 with their most appropriate method of preparation in List - 2.

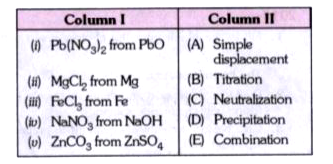
View SolutionMatch the salts given in Column I with their . method of preparation given in Column II :
View SolutionSuggest a suitable method of preparation of the salts A, B, C and D (do not describe, give only balanced chemical equations).
(i) A is a sodium salt.
(ii) B is an insoluble salt.
(iii) C is a soluble salt of copper.
(iv) D is a soluble salt of zinc.
View SolutionWrite a balanced chemical equation for the preparation of the following salt :
Copper carbonate
View SolutionWrite a balanced chemical equation for the preparation of each of the following salts:
Copper carbonate
View SolutionWrite a balanced chemical equation for the preparation of the following salt:
Copper carbonate
View SolutionIn salts of polyatomic anion, as polarising power of cation increase, thermal stability of the salt decrease and decomposed species may further undergo redox reaction
Q. Water soluble salt(x) was heated into three products A, B and C and B and C are two different paramagnetic gases. A is red in hot condition, then salt(x) is :
View Solution