Choose the correct answer from the options given below :
To increase the pH value of a neutral solution, we should add
an acid
an acid salt
an alkali
a salt
To solve the question, "To increase the pH value of a neutral solution, we should add," we can follow these steps:
1. Understand the pH Scale: The pH scale ranges from 0 to 14. A pH of 7 indicates a neutral solution. Values below 7 indicate acidity, while values above 7 indicate basicity.
2. Identify the Goal: The question asks how to increase the pH value of a neutral solution (pH = 7). To achieve this, we need to make the solution more basic.
3. Determine What to Add: To increase the pH, we should add a substance that is basic in nature. This means we need to add an alkali.
4. Consider Other Options: If we were to add an acid, the pH would decrease (become more acidic). Adding a neutral salt would not significantly change the pH, and adding an acidic salt would also lower the pH.
5. Conclusion: The correct choice to increase the pH of a neutral solution is to add an alkali.
Final Answer:
To increase the pH value of a neutral solution, we should add an alkali.
---
To increase the pH value of a neutral solution, we should add an alkali.
Topper's Solved these Questions
- STUDY OF ACIDS, BASES AND SALTSBook:ICSEChapter:STUDY OF ACIDS, BASES AND SALTSExercise:FILL IN THE BLANKS
Explore 24 Videos
- STUDY OF ACIDS, BASES AND SALTSBook:ICSEChapter:STUDY OF ACIDS, BASES AND SALTSExercise:MULTIPLE CHOICE QUESTIONS
Explore 46 Videos
- STUDY OF ACIDS, BASES AND SALTSBook:ICSEChapter:STUDY OF ACIDS, BASES AND SALTSExercise:Additional Questions for Practice
Explore 41 Videos
- SPECIMEN QUESTION PAPER (CHEMISTRY)Book:ICSEChapter:SPECIMEN QUESTION PAPER (CHEMISTRY)Exercise:Questions
Explore 40 Videos
- STUDY OF COMPOUNDS - AMMONIA Book:ICSEChapter:STUDY OF COMPOUNDS - AMMONIA Exercise:UNIT TEST PAPER 7B - AMMONIA
Explore 22 Videos
Similar Questions
Knowledge Check
Choose the correct answer from the options given below :
A compound with low boiling point, is :ASodium chlorideBCalcium chlorideCPotassium chlorideDCarbon tetrachlorideChoose the correct answer from the options given below :
The acid which can produce carbon from cane sugar, is :AConcentrated Hydrochloric acidBConcentrated Nitric acidCConcentrated Sulphuric acidDConcentrated Acetic acid
Select the correct statement from options given below.
View SolutionChoose the correct answer from the options given below :
The brown ring test is used for detection of :
View SolutionChoose the correct answer from the options given below: The catalyst used in the Contact Process is :
View SolutionMatch the columns and choose the correct answer from the options given below
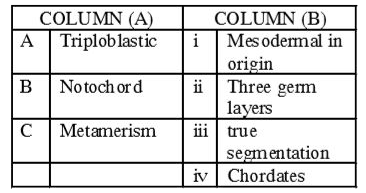
View SolutionMatch the columns and choose the correct answer from the options given below
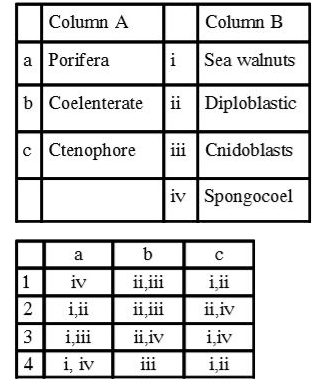
View SolutionMatch the columns and choose the correct answer from the options given below
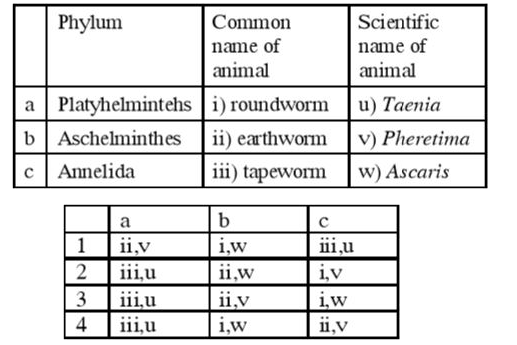
View Solution