Why don't we take silver nitrate as an electrolyte while electroplating with silver ?
Step-by-Step Solution:
1. Understanding Electroplating: Electroplating is a process that uses electrical current to reduce dissolved metal cations so that they form a coherent metal coating on an electrode. The quality of the electroplated layer depends on the electrolyte used.
2. Choosing the Electrolyte: In electroplating with silver, the choice of electrolyte is crucial. Commonly used electrolytes include silver cyanide or silver sulfate, which provide a more controlled deposition of silver.
3. Issue with Silver Nitrate: If silver nitrate is used as the electrolyte, the silver ions (Ag⁺) will be deposited onto the cathode at a much faster rate compared to other electrolytes. This rapid deposition can lead to several problems.
4. Result of Rapid Deposition: The fast deposition of silver from silver nitrate can result in a rough, uneven surface. A smooth and uniform coating is essential for aesthetic and functional purposes in electroplating.
5. Conclusion: Therefore, silver nitrate is not used as an electrolyte for electroplating with silver because it leads to a rapid deposition of silver, which is not smooth and uniform.
Final Answer:
Silver nitrate is not used as an electrolyte for electroplating with silver because it causes the deposition of silver to occur very quickly, resulting in a rough and uneven surface.
---
Silver nitrate is not used as an electrolyte for electroplating with silver because it causes the deposition of silver to occur very quickly, resulting in a rough and uneven surface.
Topper's Solved these Questions
- ELECTROLYSISBOOK - ICSECHAPTER - ELECTROLYSISEXERCISE - WORK SHEET -4 (complete the following : )
5 Videos
- ELECTROLYSISBOOK - ICSECHAPTER - ELECTROLYSISEXERCISE - ADDITIONAL QUESTIONS FOR PRACTICE
49 Videos
- ELECTROLYSISBOOK - ICSECHAPTER - ELECTROLYSISEXERCISE - WORK SHEET -3 (Complete the following table with respect to extraction of aluminium : )
1 Video
- CHEMISTRY-2015BOOK - ICSECHAPTER - CHEMISTRY-2015EXERCISE - SECTION-II (40 Marks) Attempt any four questions from this Section.
49 Videos
- METALLURGYBOOK - ICSECHAPTER - METALLURGYEXERCISE - UNIT TEST PAPER 6 - Metallurgy (Select the correct answer from the list A,B,C & given in each statement.)
5 Videos
Similar Questions
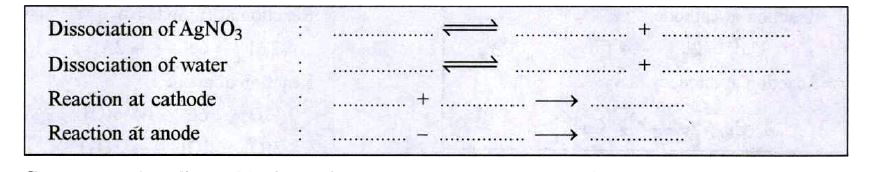
Electrolysis of Silver nitrate using silver electrodes. Complete the table
View SolutionWhat is the difference between :
Conductivity of copper metal and of CuSO4 solution ?
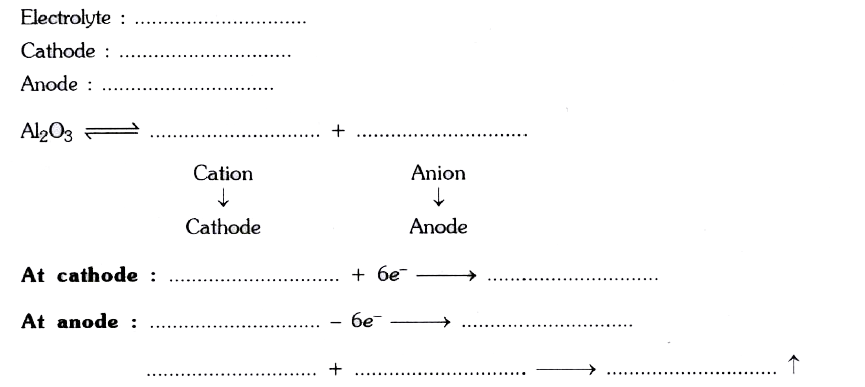
View SolutionComplete the following table with respect to extraction of aluminium :
View SolutionArrange the following as per the instructions given in the brackets:
Cs, Na, Li, K, Rb (increasing order of metallic character).
View SolutionThe oxide of the metal which reacts with both acids and alkalis-to give salt & water
(a) MgO
(b) CuO
(c) Al2O3
(d) K2O
View SolutionThe electrolytes usually used in the electroplating of gold and silver, respectively, are
View SolutionIf a metal is to be electroplated with silver, what would happen if a solution of silver nitrate alone is used as an electrolyte ? What is the preferable electrolyte and why is it used ?
View SolutionThe electrolyte for the electroplating an article with silver is
View SolutionA metal object is to be electroplated with silver .The electroplated with silver . The electrolyte selected is sodium argentocyanide . What type of salt is sodium argentocyanide ?
View SolutionA metal object is to be electroplated with silver .The electroplated with silver . The electrolyte selected is sodium argentocyanide .
Why is it preferred to use silver nitrate as an electrolyte ?
View SolutionA metal object is to be electroplated with silver . The electrolyte selected is
View Solution....... is the electrolyte used for electroplating an iron spoon with silver.
View SolutionElectrolyte not used for electroplating with silver is .......
View SolutionGive reason :
For electroplating with silver, silver nitrate is not used as electrolyte.
View SolutionA metal article is to be electroplated with silver. The electrolyte selected is sodium argentocyanide. Why is it preferred to silver nitrate as an electrolyte?
View Solution
