Calcium, Copper, Lead, Aluminium, Zinc, Chromium, Magnesium and Iron. Choose the major metals from the list given above to make the following alloys :
Brass
Step by step video solution for Calcium, Copper, Lead, Aluminium, Zinc, Chromium, Magnesium and Iron. Choose the major metals from the list given above to make the following alloys : Brass by Chemistry experts to help you in doubts & scoring excellent marks in Class 10 exams.
Updated on:21/07/2023Topper's Solved these Questions
- METALLURGYBook:ICSEChapter:METALLURGYExercise:QUESTIONS
Explore 127 Videos
- METALLURGYBook:ICSEChapter:METALLURGYExercise:QUESTIONS FOR PRACTICE
Explore 53 Videos
- METALLURGYBook:ICSEChapter:METALLURGYExercise:ADDITIONAL QUESTIONS FOR PRACTICE
Explore 39 Videos
- ELECTROLYSISBook:ICSEChapter:ELECTROLYSISExercise:Assertion and Reason Based Questions
Explore 4 Videos
- MODEL PAPER -1Book:ICSEChapter:MODEL PAPER -1Exercise:SECTION -II
Explore 48 Videos
Similar Questions
Calcium, Copper, Lead, Aluminium, Zinc, Chromium, Magnesium and Iron. Choose the major metals from the list given above to make the following alloys :
Stainless steel
View SolutionCalcium, Copper, Lead, Aluminium, Zinc, Chromium, Magnesium, Iron Choose the major metals from the list given above to make the following alloys : (i) Stainless steel. (ii) Brass.
View SolutionChoosing the substances from the list given: dil Sulphuric acid, Copper, Iron, Sodium, Copper [II} carbonate, Sodium carbonate, Sodium chloride, Zinc nitrate-
Write balanced equations for the reactions which would be used in the laboratory to obtain the following salts. [i] Sodium sulphate [ii] Zinc carbonate [iii] Copper [II] sulphate [iv] Iron (II) sulphate
View SolutionChoose the correct answer from the options A,B,C & D given : The reason for using aluminium in the alloy duralumin is :
View SolutionChoosing the substances from the list given below, write balanced chemical equations for the reactions which would be used in the laboratory to obtain the following salts:
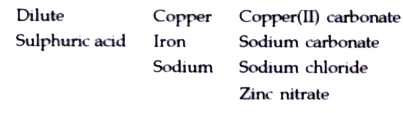
Zinc carbonate
View SolutionFill in the blanks choosing appropriate word/ words from those given in the brackets : (gravity separation, magnetic separation, absence, presence, CuFeS2, Cu2S, froth flotation, aluminium, iron, smelting, pure, impure, mines, copper, silver, sulfide, zinc, chromium nickel, tin, blister copper, Cus, Fes, thermal, electrical, argentite or silver glance (Ag2S), cuprous oxide, cuprous sulfide.)
The process of reduction of metal oxides with carbon is called.........
View SolutionFill in the blanks choosing appropriate word/ words from those given in the brackets : (gravity separation, magnetic separation, absence, presence, CuFeS2, Cu2S, froth flotation, aluminium, iron, smelting, pure, impure, mines, copper, silver, sulfide, zinc, chromium nickel, tin, blister copper, Cus, Fes, thermal, electrical, argentite or silver glance (Ag2S), cuprous oxide, cuprous sulfide.)
The process of reduction of metal oxides with carbon is called.........
View SolutionFill in the blanks choosing appropriate word/ words from those given in the brackets : (gravity separation, magnetic separation, absence, presence, CuFeS2, Cu2S, froth flotation, aluminium, iron, smelting, pure, impure, mines, copper, silver, sulfide, zinc, chromium nickel, tin, blister copper, Cus, Fes, thermal, electrical, argentite or silver glance (Ag2S), cuprous oxide, cuprous sulfide.)
The process of reduction of metal oxides with carbon is called.........
View SolutionFill in the blanks choosing appropriate word/ words from those given in the brackets : (gravity separation, magnetic separation, absence, presence, CuFeS2, Cu2S, froth flotation, aluminium, iron, smelting, pure, impure, mines, copper, silver, sulfide, zinc, chromium nickel, tin, blister copper, Cus, Fes, thermal, electrical, argentite or silver glance (Ag2S), cuprous oxide, cuprous sulfide.)
Mining is the process of taking out the ore from the ...........
View SolutionFill in the blanks choosing appropriate word/ words from those given in the brackets : (gravity separation, magnetic separation, absence, presence, CuFeS2, Cu2S, froth flotation, aluminium, iron, smelting, pure, impure, mines, copper, silver, sulfide, zinc, chromium nickel, tin, blister copper, Cus, Fes, thermal, electrical, argentite or silver glance (Ag2S), cuprous oxide, cuprous sulfide.)
The removal of gangue from the ores is called ......
View Solution