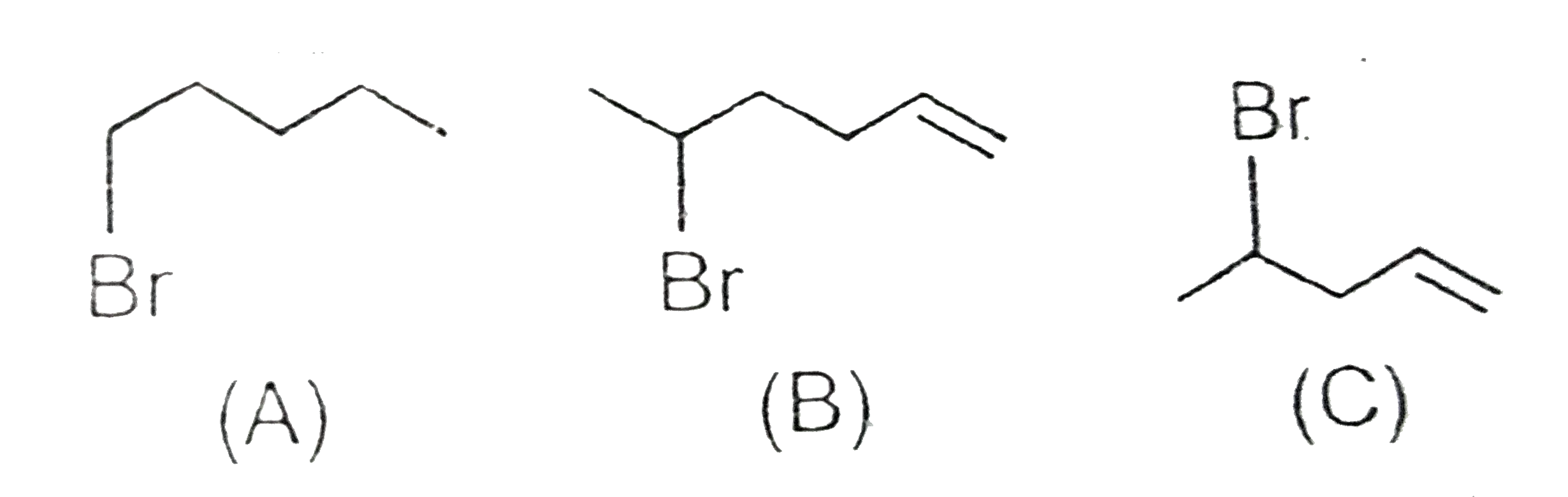
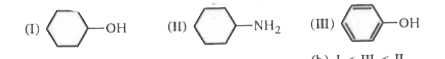
Rank the following compounds in order of increasing acidity.

Text Solution
Alcohols are less acidic than phenols, and phenols are less acidic than carboxylic acids. An electron-withdrawing group increases the acidity of a phenol relative to phenol itself. Thus the order of increasing acidity among these examples is cyclohexanol < phenol < 4-nitrophenol < benzoic acid.
|
Similar Questions
Knowledge Check
Rank then following compounds in order of increasing acidity (weakest acid first )numbers given are 1,2 and 3 respectively.
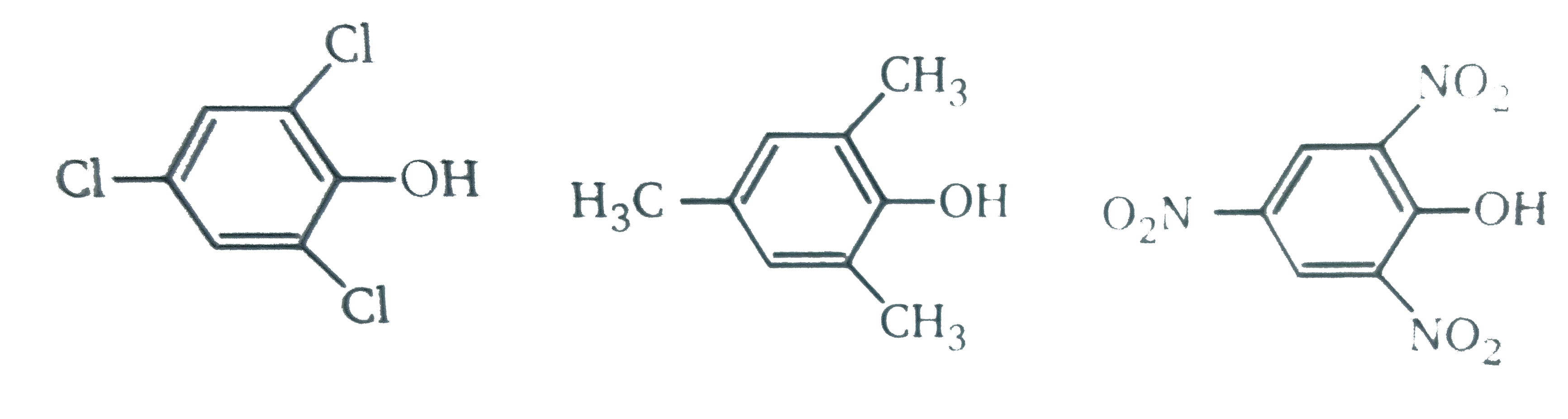 A2<3<1B3<1<2C1<2<3D2<1<3
A2<3<1B3<1<2C1<2<3D2<1<3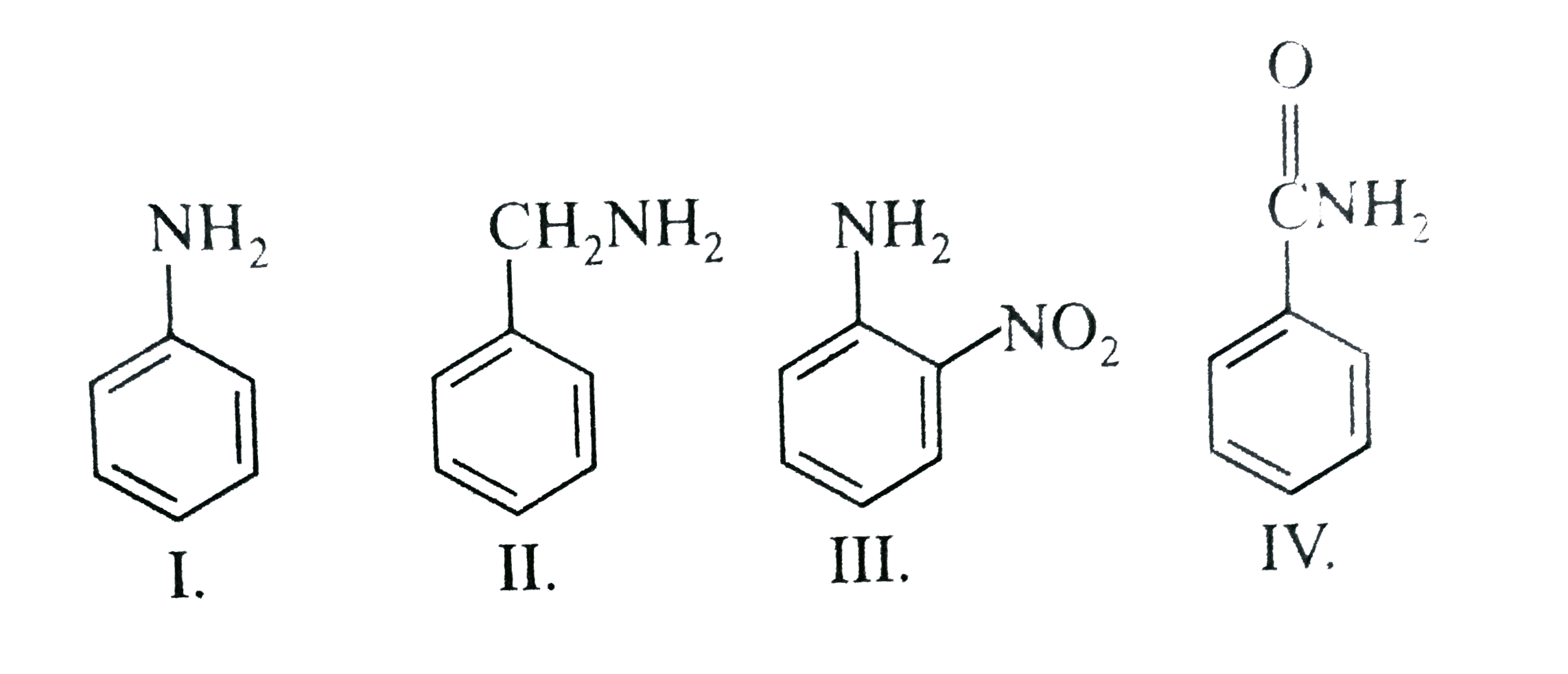
Rank the following compounds in order of increasing basicity (weakest → strongest)AIV<II<I<IIIBIV<III<I<IICIV<I<III<IIDII<I<III<IVRank the following compounds in order of increasing basic strength. (weakest→strongest)
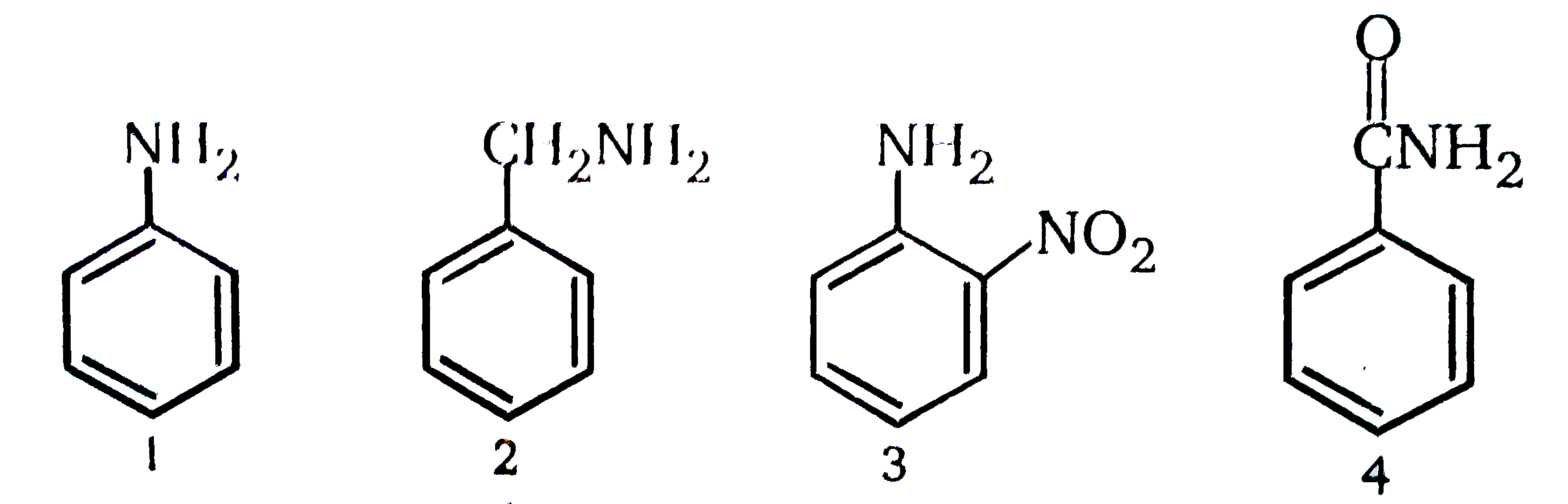 A4<2<1<3B4<3<1<2C4<1<3<2D2<1<3<4
A4<2<1<3B4<3<1<2C4<1<3<2D2<1<3<4
Rank the following compounds in order of decreasing acid strength (most acidic → least acidic).
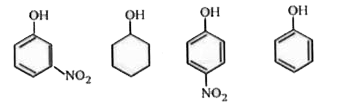
View SolutionRank the following compounds in order of decreasing acid strength (most acidit → least acidic )
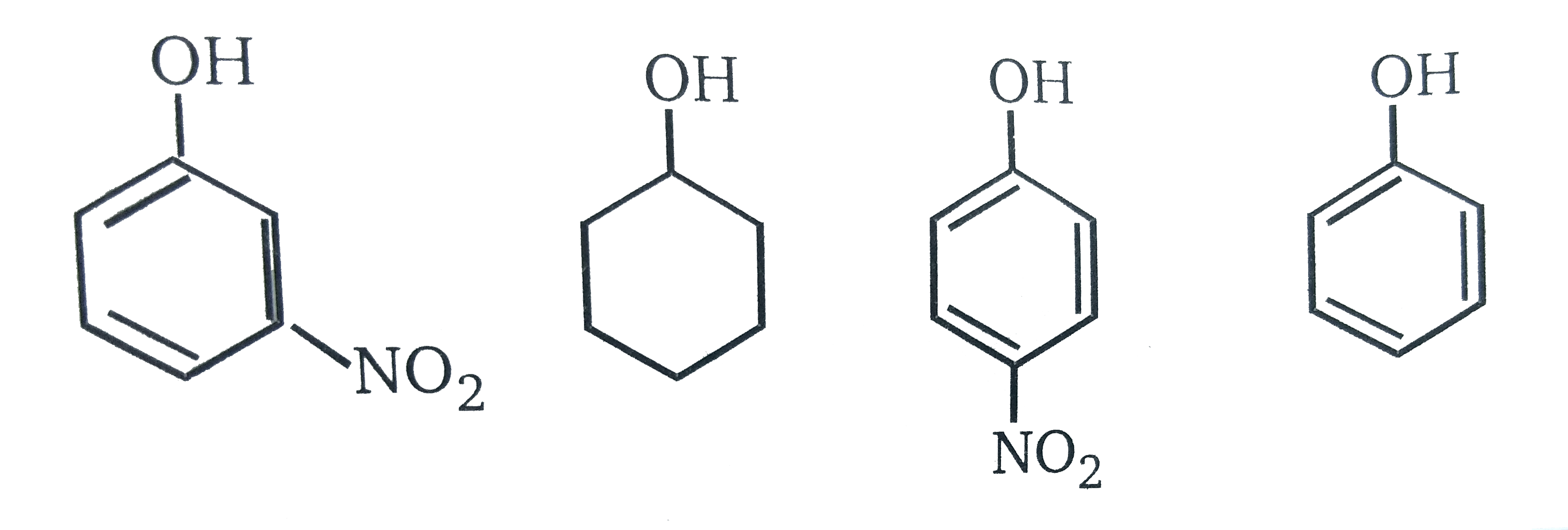
View SolutionArrange the following acid in order of increasing acidity
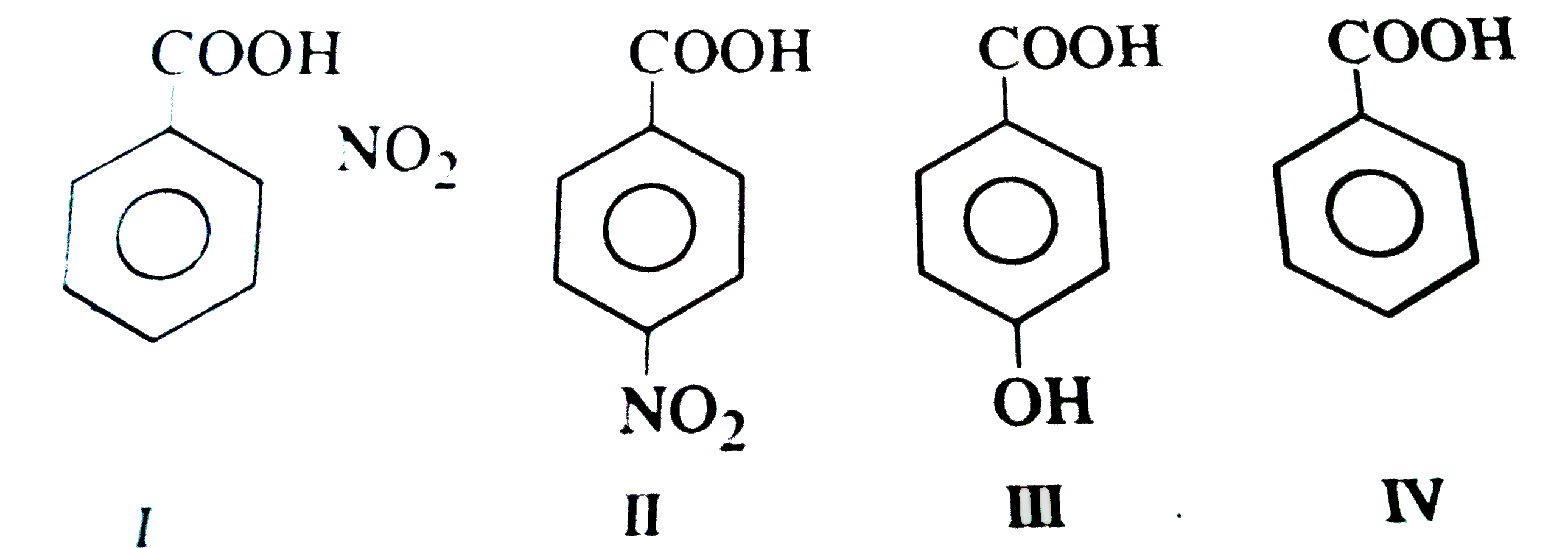
View SolutionArrange the following acids in order of their increasing acidity
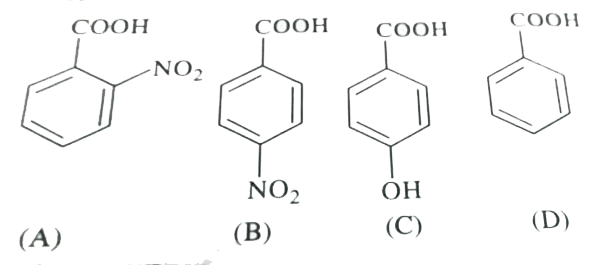
View SolutionRank in the order of increasing acidity.
View Solution