The correct order of N-compounds in its decreasing order of oxidation states is
HNO3,NO,N2,NH4Cl
HNO3,NO,NH4Cl4,N2
HNO3,NH4Cl,NO,N2
NH4Cl,N2,NO,HNO3
The correct Answer is:A
To determine the correct order of nitrogen compounds in decreasing order of oxidation states, we will analyze each compound step by step.
Step 1: Determine the oxidation state of nitrogen in HNO3 (Nitric Acid)
1. Let the oxidation state of nitrogen be x.
2. The oxidation state of hydrogen (H) is +1, and for each oxygen (O), it is -2.
3. The formula for HNO3 can be set up as follows:
x+1+3(−2)=0
4. Simplifying this gives:
x+1−6=0⟹x−5=0⟹x=+5
Step 2: Determine the oxidation state of nitrogen in NO (Nitric Oxide)
1. Let the oxidation state of nitrogen be x.
2. The oxidation state of oxygen is -2.
3. The formula for NO can be set up as follows:
x+(−2)=0
4. Simplifying this gives:
x−2=0⟹x=+2
Step 3: Determine the oxidation state of nitrogen in N2 (Elemental Nitrogen)
1. In elemental form, the oxidation state of nitrogen is 0.
x=0
Step 4: Determine the oxidation state of nitrogen in Ammonium Chloride (NH4Cl)
1. Let the oxidation state of nitrogen be x.
2. The oxidation states of hydrogen are +1 (for 4 hydrogens) and chlorine is -1.
3. The formula for NH4Cl can be set up as follows:
x+4(1)+(−1)=0
4. Simplifying this gives:
x+4−1=0⟹x+3=0⟹x=−3
Step 5: Compile the oxidation states
- HNO3: +5
- NO: +2
- N2: 0
- NH4Cl: -3
Step 6: Arrange in decreasing order
The decreasing order of oxidation states of nitrogen compounds is:
1. HNO3: +5
2. NO: +2
3. N2: 0
4. NH4Cl: -3
Thus, the correct order of nitrogen compounds in decreasing order of oxidation states is:
HNO3 > NO > N2 > NH4Cl
Final Answer
The correct order of nitrogen compounds in decreasing order of oxidation states is:
HNO3, NO, N2, NH4Cl
---
1. Let the oxidation state of nitrogen be x.
2. The oxidation state of hydrogen (H) is +1, and for each oxygen (O), it is -2.
3. The formula for HNO3 can be set up as follows:
x+1+3(−2)=0
4. Simplifying this gives:
x+1−6=0⟹x−5=0⟹x=+5
1. Let the oxidation state of nitrogen be x.
2. The oxidation state of oxygen is -2.
3. The formula for NO can be set up as follows:
x+(−2)=0
4. Simplifying this gives:
x−2=0⟹x=+2
1. In elemental form, the oxidation state of nitrogen is 0.
x=0
1. Let the oxidation state of nitrogen be x.
2. The oxidation states of hydrogen are +1 (for 4 hydrogens) and chlorine is -1.
3. The formula for NH4Cl can be set up as follows:
x+4(1)+(−1)=0
4. Simplifying this gives:
x+4−1=0⟹x+3=0⟹x=−3
- HNO3: +5
- NO: +2
- N2: 0
- NH4Cl: -3
The decreasing order of oxidation states of nitrogen compounds is:
1. HNO3: +5
2. NO: +2
3. N2: 0
4. NH4Cl: -3
HNO3 > NO > N2 > NH4Cl
The correct order of nitrogen compounds in decreasing order of oxidation states is:
HNO3, NO, N2, NH4Cl
Topper's Solved these Questions
- REDOX REACTIONS (OXIDATION AND REDUCTION)BOOK - ICSECHAPTER - REDOX REACTIONS (OXIDATION AND REDUCTION)EXERCISE - TRUE OR FALSE. TYPE QUESTIONS (State whether the following statements are True (T) or False (F):)
17 Videos
- REDOX REACTIONS (OXIDATION AND REDUCTION)BOOK - ICSECHAPTER - REDOX REACTIONS (OXIDATION AND REDUCTION)EXERCISE - FILL IN THE BLANKS. TYPE QUESTIONS
12 Videos
- REDOX REACTIONS (OXIDATION AND REDUCTION)BOOK - ICSECHAPTER - REDOX REACTIONS (OXIDATION AND REDUCTION)EXERCISE - ESSAY (LONG ANSWER) TYPE QUESTIONS
8 Videos
- ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES BOOK - ICSECHAPTER - ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES EXERCISE - NCERT TEXT-BOOK EXERCISES (WITH HINTS AND SOLUTIONS )
72 Videos
- SAMPLE QUESTION PAPERBOOK - ICSECHAPTER - SAMPLE QUESTION PAPEREXERCISE - Question
69 Videos
Similar Questions
Knowledge Check
Arrange the following compounds in decreasing order of acidity
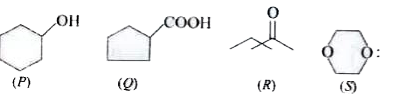 AP>Q>S>RBQ>R>S>PCQ>P>R>SDR>P>Q>S
AP>Q>S>RBQ>R>S>PCQ>P>R>SDR>P>Q>SArrange the following compounds in decreasing order of acidity
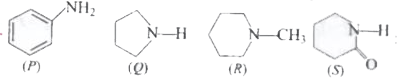 AP>Q>R>SBR>Q>P>SCS>P>R>QDS>P>Q>R
AP>Q>R>SBR>Q>P>SCS>P>R>QDS>P>Q>RArrange the following compounds in decreasing order of acidity
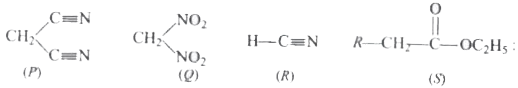 AP>Q>R>SBQ>P>R>SCQ>R>P>SDS>Q>P>R
AP>Q>R>SBQ>P>R>SCQ>R>P>SDS>Q>P>R
Arrange basicity of given compounds in decreasing order
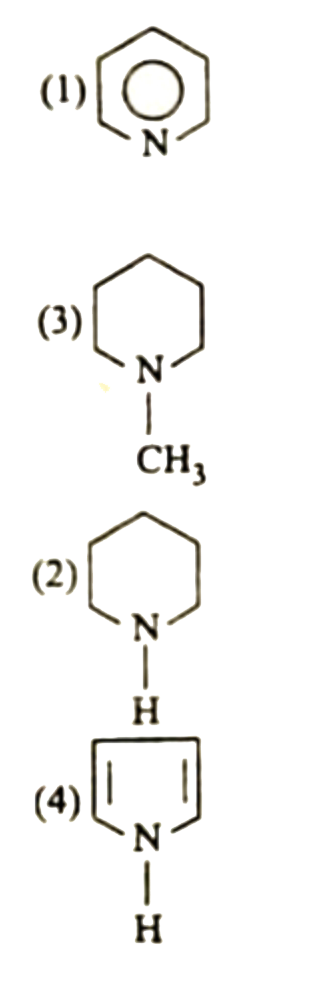
View SolutionArrange pH of the given compounds in decreasing order :
View SolutionArrange the following compounds in decreasing order of acidity
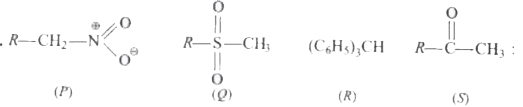
View SolutionArrange the following compounds in decreasing order of acidity
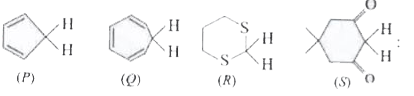
View SolutionArrange the following compounds in decreasing order of acidity :
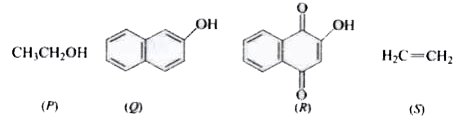
View Solution

