Name the type of reactions for the following chemical equations.
CuO+H2→Cu+H2O
To determine the type of reaction for the given chemical equation CuO+H2→Cu+H2O, we will analyze the components and the changes occurring during the reaction.
1. Identify the Reactants and Products:
- The reactants are Copper(II) oxide (CuO) and Hydrogen gas (H2).
- The products are Copper metal (Cu) and Water (H2O).
2. Analyze the Changes in Oxidation States:
- In CuO, Copper (Cu) is in the +2 oxidation state (Cu^2+), and Oxygen (O) is in the -2 oxidation state (O^2-).
- In the products, Copper (Cu) is in the 0 oxidation state (Cu^0) and in Water (H2O), Oxygen (O) is in the -2 oxidation state (O^2-).
- Here, Copper is reduced from +2 to 0 (gaining electrons), while Hydrogen is oxidized from 0 to +1 (losing electrons).
3. Determine the Type of Reaction:
- Since there is a transfer of electrons where one substance is reduced (CuO) and another is oxidized (H2), this reaction involves both reduction and oxidation processes occurring simultaneously.
- Therefore, this type of reaction is classified as a Redox reaction (a combination of reduction and oxidation).
Final Answer:
The type of reaction for the equation CuO+H2→Cu+H2O is a Redox reaction.
---
- The reactants are Copper(II) oxide (CuO) and Hydrogen gas (H2).
- The products are Copper metal (Cu) and Water (H2O).
- In CuO, Copper (Cu) is in the +2 oxidation state (Cu^2+), and Oxygen (O) is in the -2 oxidation state (O^2-).
- In the products, Copper (Cu) is in the 0 oxidation state (Cu^0) and in Water (H2O), Oxygen (O) is in the -2 oxidation state (O^2-).
- Here, Copper is reduced from +2 to 0 (gaining electrons), while Hydrogen is oxidized from 0 to +1 (losing electrons).
- Since there is a transfer of electrons where one substance is reduced (CuO) and another is oxidized (H2), this reaction involves both reduction and oxidation processes occurring simultaneously.
- Therefore, this type of reaction is classified as a Redox reaction (a combination of reduction and oxidation).
The type of reaction for the equation CuO+H2→Cu+H2O is a Redox reaction.
Topper's Solved these Questions
Similar Questions
Give reasons :
Sodium is kept in an inert solvent.
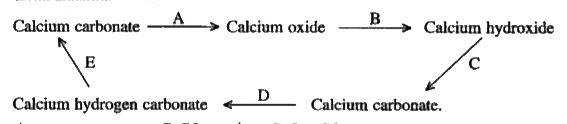
View SolutionWrite equation for each conversion A to E.
View SolutionCopper sulphate crystals are heated strongly in a hard glass test tube. Write three observations noticed.
View Solutionनिम्न में से कौन-सी अभिक्रिया संभव है और क्यों ?
(i) SnO+H2→Sn+H2O
(ii) CuO+H2→Cu+H2O
View SolutionIn the reaction CuO+H2→Cu+H2O , cupric oxide (CuO) is getting reduced to Cu.
View SolutionClassify the reactions into different type.
2KClO3(s)Δ−−→2KCl(aq)+3O2(g)
View Solutionनिम्नलिखित अभिक्रियाओं को अपचयोपचय अभिक्रियाओं के रूप में औचित्य स्थापित करने का प्रयास करें -
CuO(s)+H2(g)→Cu(s)+H2O(g)
View SolutionCuO+H2→Cu+H2O किस प्रकार की अभिक्रिया है
View SolutionJustify the following reactions are redox reactions :
CuO(s)+H2(g)→Cu(s)+H2O(g)
View SolutionBalance the reaction by oxidation number method
CuO+NH3→Cu+N2+H2O
View SolutionThe balance chemical equation for the reaction Cu+HNO3→Cu(NO3)2+NO+H2O involves
View SolutionIdentify the substances that are oxidized and the substances that are reduced in the following reactions :
CuO(s)+H2(g)→Cu(s)+H2O(l).
View SolutionIdentify the reducing agent and oxidizing agent in the following reactions : CuO+H_(2) toCu+H_(2)O
View SolutionIdentify the substances that are oxidised and the substances that are reduced in the following reactions :
CuO(s)+H2(g)→Cu(s)+H2O(l)
View SolutionName of type of chemical reaction represented by the following equation : (i) CaO+H_(2)O rarr Ca(OH)_2
View Solution
