A black residue is formed when sugar is heated. …………………..
Step-by-Step Solution:
1. Identify the Chemical Composition of Sugar:
- The chemical formula of sugar (sucrose) is C₁₂H₂₂O₁₁.
2. Understand the Heating Process:
- When sugar is heated, it undergoes a chemical change. The heat causes the sugar to decompose.
3. Decomposition of Sugar:
- Upon heating, sugar breaks down into its constituent elements. Specifically, it separates into carbon (C) and water (H₂O).
4. Chemical Reaction:
- The chemical reaction can be represented as:
C12H22O11→12C+11H2O
- This shows that for every molecule of sugar, 12 atoms of carbon and 11 molecules of water are produced.
5. Formation of Black Residue:
- The carbon produced during this reaction is in solid form and appears as a black residue.
6. Conclusion:
- Therefore, the black residue formed when sugar is heated is carbon.
Final Answer:
The black residue formed when sugar is heated is carbon.
---
- The chemical formula of sugar (sucrose) is C₁₂H₂₂O₁₁.
- When sugar is heated, it undergoes a chemical change. The heat causes the sugar to decompose.
- Upon heating, sugar breaks down into its constituent elements. Specifically, it separates into carbon (C) and water (H₂O).
- The chemical reaction can be represented as:
C12H22O11→12C+11H2O
- This shows that for every molecule of sugar, 12 atoms of carbon and 11 molecules of water are produced.
- The carbon produced during this reaction is in solid form and appears as a black residue.
- Therefore, the black residue formed when sugar is heated is carbon.
The black residue formed when sugar is heated is carbon.
Topper's Solved these Questions
- LANGUAGE OF CHEMISTRY Book:ICSEChapter:LANGUAGE OF CHEMISTRY Exercise:OBJECTIVE TYPE QUESTIONS (MULTIPLE CHOICE QUESTIONS)
Explore 6 Videos
- LANGUAGE OF CHEMISTRY Book:ICSEChapter:LANGUAGE OF CHEMISTRY Exercise:OBJECTIVE TYPE QUESTIONS (FILL IN THE BLANKS)
Explore 5 Videos
- ELEMENTS COMPOUNDS AND MIXTURES. Book:ICSEChapter:ELEMENTS COMPOUNDS AND MIXTURES. Exercise:OBJECTIVE TYPE QUESTIONS (MULTIPLE CHOICE QUESTIONS)
Explore 5 Videos
- MATTER AND ITS COMPOSITIONBook:ICSEChapter:MATTER AND ITS COMPOSITIONExercise:EXERCISE
Explore 19 Videos
Similar Questions
Knowledge Check
Which of the following products is formed when adipic acid is heated ?
A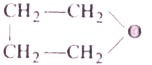 B
B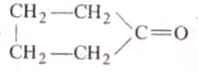 C
C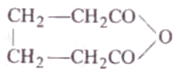 D
D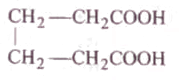
Which one is formed when sodium phenoxide is heated with ethyl iodide ?
APhenetoleBEthyl phenyl alcoholCPhenolDNone of these.
Which compound is formed when sodium is heated in oxygen ?
View SolutionThe action of heat on the blue crystalline solid L gives a reddish brown gas M, a gas which re-lights a glowing splint and leaves a black residue. When gas N, which has a rotten egg smell, is passed through a solution of La black precipitate is formed.
Write the equation for the action of heat on L.
View SolutionThe insoluble residue formed when solutions of two compounds react with each other
View SolutionThe action of heat on the blue crystalline solid L gives a reddish brown gas M, a gas which re-lights a glowing splint and leaves a black residue. When gas N, which has a rotten egg smell, is passed through a solution of La black precipitate is formed.
Write the equation for the reaction between the solution of L and the gas N.
View SolutionName the products formed when copper is heated with concentrated sulphuric acid.
View SolutionOn strongly heating, a blue salt leaves a black residue. Which of the following cations can be present in the salt?
View Solution