CH3CONH2 is a weaker base than CH3CH2NH2.
CH3−O∣∣C− group in CH3CONH2, withdraws electron pair on the nitrogen through resonance.
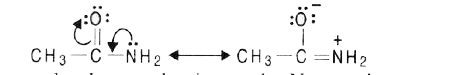
As a result, electron density on the N-atom in acetamide decreases. On the other hand, in ethylamine, due to +I effect of the ethyl group, electron density on the N-atom increases. Consequently, CH3CONH2 is a weaker base than CH3CH2NH2.
Topper's Solved these Questions
- ORGANIC COMPOUNDS CONTAINING NITROGENBOOK - KALYANI PUBLICATIONCHAPTER - ORGANIC COMPOUNDS CONTAINING NITROGENEXERCISE - EXERCISE (PART-II DESCRIPTIVE QUESTIONS- SHORT ANSWER QUESTIONS )
21 Videos
- ORGANIC COMPOUNDS CONTAINING NITROGENBOOK - KALYANI PUBLICATIONCHAPTER - ORGANIC COMPOUNDS CONTAINING NITROGENEXERCISE - EXERCISE (PART-II DESCRIPTIVE QUESTIONS- LONG ANSWER QUESTIONS )
12 Videos
- ORGANIC COMPOUNDS CONTAINING NITROGENBOOK - KALYANI PUBLICATIONCHAPTER - ORGANIC COMPOUNDS CONTAINING NITROGENEXERCISE - EXERCISE (PART-I OBJECTIVE QUESTIONS)
67 Videos
- d- AND- f- BLOCK ELEMENTSBOOK - KALYANI PUBLICATIONCHAPTER - d- AND- f- BLOCK ELEMENTSEXERCISE - EXERCISE (PART-II) (DESCRIPTIVE QUESTIONS)(VERY SHORT ANSWER QUESTIONS)
85 Videos
- P-BLOCK ELEMENTSBOOK - KALYANI PUBLICATIONCHAPTER - P-BLOCK ELEMENTSEXERCISE - ISC EXAMINATION QUESTIONS(PART-I)(OBJECTIVE QUESTIONS)(THE CORRECT ALTERNATIVE FROM THE CHOICES GIVEN :)
6 Videos
Similar Questions
When acetamide is treated with Br2 and an alkali, a primary amine containing one carbon atom less than the starting amide is formed. Name the reaction and write the balanced equation.
View SolutionHow will you prepare :
a pure sample of a primary amine from a primary alkyl halide
View SolutionR2NH+HNO2→………..
View SolutionWhy are transition elements so named ?
View SolutionWhen hydrogen sulfide reacts with sulfur dioxide to give sulfur and water,
View SolutionWhich of the following should be most volatile?
I) CH3CH2CH2NH2II)(CH3)3NIII)
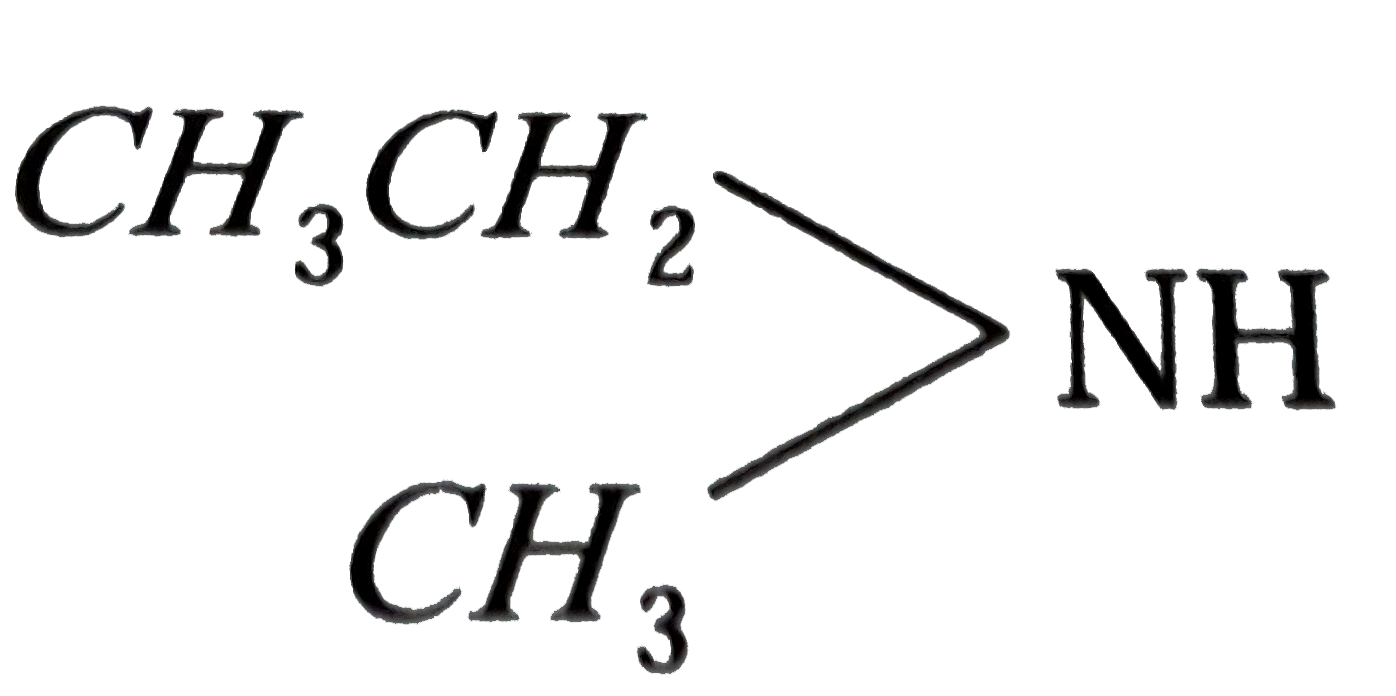
IV) CH3CH2CH3
View SolutionCH_3 CH_2 NH_2 is soluble in
View SolutionCH3−CH2−CH2−CONH2 यौगिक के IUPAC नाम लिखें-
View Solutionनिम्नलिखित यौगिकों के आई०यू०पी०ए०सी० नाम लिखिएCH3−CH2−CH(NH2)−CH3
View SolutionCH3−CH2−CH∣CH3−NH2 का IUPAC नाम है
View SolutionCH3−CH2−CH∣CH3−NH2 का IUPAC नाम है
View SolutionThe IUPAC name of the compound, CH3−CH2−CH(CH3)−CONH2 is:
View SolutionCH3CONH2,Br2 व KOH उत्पाद के रूप में CH3NH2 देते हैं अभिक्रिया के मध्यवर्ती है

View SolutionWhich amine in each of the following pairs is a stronger base? Give reasons.
CH3CH2CH2NH2orCH3NHCH2CH3
View SolutionCH3CONH2 is a weaker base than CH3CH2NH2.
View Solution

