What type of electrons get displaced in
(i) inductive effect
(ii) electromeric effect?
Video Solution
Text Solution
σ-electrons (ii) π-electrons
|
Topper's Solved these Questions
- ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES Book:ICSEChapter:ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES Exercise:SHORT ANSWER TYPE QUESTIONS
Explore 110 Videos
- ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES Book:ICSEChapter:ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES Exercise:ESSAY ( LONG ANSWER ) TYPE QUESTIONS
Explore 42 Videos
- ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES Book:ICSEChapter:ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES Exercise:REVIEW EXERCISES
Explore 176 Videos
- HYDROGENBook:ICSEChapter:HYDROGENExercise:NCERT TEXT-BOOK EXERCISE (With Hints and Solutions)
Explore 53 Videos
- REDOX REACTIONS (OXIDATION AND REDUCTION)Book:ICSEChapter:REDOX REACTIONS (OXIDATION AND REDUCTION)Exercise:NCERT TEXT-BOOK. EXERCISES ((With Hints and Solutions)
Explore 69 Videos
Similar Questions
Knowledge Check
Inductive effect involves
Adisplacement off σ-electrons resulting in polarisationBdisplacement of π-electrons resulting in polarisationCdelocalisation of σ-electronsDdelocalisation of π-electrons.Which of the following exhibit electromeric effect ?
AAlkanesBAldehydesCAlkyl halidesDAlkyl amines
Compare inductive effect with mesomeric effect.
View SolutionWhat is inductive effect ? Explain
View SolutionDifferentiate between inductive and electromeric effect
View SolutionWhat type of effects are involved in the following reactions ?
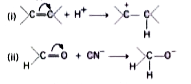
View SolutionInductive effect is a polarisation of a
View SolutionWhat is elastic after-effect?
View Solution
