Explain the terms Inductive and Electromeric effects. Which electron displacement effect explains the following correct orders of acidity of the carboxylic acids?
(a) Cl3CCOOH>Cl2CHCOOH>ClCH2COOH
(b) CH3CH2COOH>(CH3)2CHCOOH>(CH3)3C.COOH
-I-effect as shown below:
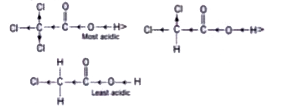
-l-effect is maximum in CI3C.COOHdue to the presence of three-Cl atoms. The effect and hence the proton releasing tendency decreases with decrease in the number of CI atoms. This explains the above order of acidity.
Topper's Solved these Questions
- ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES BOOK - ICSECHAPTER - ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES EXERCISE - ASSERTION - REASON TYPE QUESTIONS
11 Videos
- HYDROGENBOOK - ICSECHAPTER - HYDROGENEXERCISE - NCERT TEXT-BOOK EXERCISE (With Hints and Solutions)
53 Videos
- REDOX REACTIONS (OXIDATION AND REDUCTION)BOOK - ICSECHAPTER - REDOX REACTIONS (OXIDATION AND REDUCTION)EXERCISE - NCERT TEXT-BOOK. EXERCISES ((With Hints and Solutions)
69 Videos
Similar Questions
Knowledge Check
Which of the following is the correct orderr of acidity of carboxylic acids?
(i) Cl3CCOOH>Cl2CHCOOH>ClCH2COOH
(ii) CH3CH2COOH>(CH3)2CHCOOH>(CH3)3CCOOH
(iii) F2CHCOOH>FCH2COOH>ClCH2COOHA(i) and (ii)B(ii) and (iii)C(i) and (iii)D(i), (ii) and (iii)
Arrange the following compounds in decreasing order of acidity.
(i) ClCH2CH2CH2COOH
(ii) CH3CHClCH2COOH
(iii) CH3CH2CHClCOOH
View SolutionArrange the following compounds in an increasing order of their acid strengths :
(CH3)2CHCOOH,CH3CH2CH(Br)COOH,CH3CH(Br)CH2COOH
View SolutionArrange the following compounds in increasing order of their acid strength.
CH3CH2CH(Br)COOH,CH3CH(Br)CH2COOH,(CH3)2CHCOOH,CH3CH2CH2COOH
View SolutionArrange the following compounds in increasing order of their acid strengths:
(CH3)2CHCOOH,CH3CH2CH(Br)COOH,
CH3CH(Br)CH2COOH
View SolutionArrange the following compounds in an increasing order of their acid strengths :
(CH3)2CHCOOH,CH3CH2CH(Br)COOH,CH3CH(Br)CH2COOH
View SolutionArrange the following compounds in an increasing order of their acid strengths :
(CH3)2CHCOOH,CH3CH2CH(Br)COOH,CH3CH(Br)CH2COOH
View SolutionArrange the following as stated :
'Increasing order of acidic strength'.
ClCH2COOH,CH3CH2COOH,ClCH2CH2COOH,(CH3)2CHCOOH,CH3COOH.
View Solution

