Select the correct word or formula from the same give in bracket :
The acid which is not an oxidising agent [Conc HNO3/ConcHCl/Conc.H2SO4]
To solve the question, we need to identify which of the given acids is not an oxidizing agent. The options provided are:
1. Concentrated Nitric Acid (Conc HNO₃)
2. Concentrated Hydrochloric Acid (Conc HCl)
3. Concentrated Sulfuric Acid (Conc H₂SO₄)
1. Understanding Oxidizing Agents:
- An oxidizing agent is a substance that can accept electrons from another species, causing the other species to be oxidized while itself is reduced.
2. Analyzing Concentrated Nitric Acid (Conc HNO₃):
- Concentrated nitric acid is known to be a strong oxidizing agent. The nitrogen in nitric acid has an oxidation state of +5, and it can be reduced to lower oxidation states (like +4, +3, +2, or +1), thus it can oxidize other substances.
3. Analyzing Concentrated Sulfuric Acid (Conc H₂SO₄):
- Concentrated sulfuric acid is also a strong oxidizing agent. The sulfur in sulfuric acid has an oxidation state of +6 and can be reduced to lower oxidation states. Therefore, it can also act as an oxidizing agent.
4. Analyzing Concentrated Hydrochloric Acid (Conc HCl):
- Concentrated hydrochloric acid contains chloride ions (Cl⁻), which are already in their lowest oxidation state (-1). Since chloride cannot be further reduced, concentrated hydrochloric acid does not have the ability to oxidize other substances. Thus, it does not act as an oxidizing agent.
5. Conclusion:
- Based on the analysis, the acid that is not an oxidizing agent is Concentrated Hydrochloric Acid (Conc HCl).
Final Answer:
The acid which is not an oxidizing agent is Concentrated Hydrochloric Acid (Conc HCl).
---
2. Concentrated Hydrochloric Acid (Conc HCl)
3. Concentrated Sulfuric Acid (Conc H₂SO₄)
- An oxidizing agent is a substance that can accept electrons from another species, causing the other species to be oxidized while itself is reduced.
- Concentrated nitric acid is known to be a strong oxidizing agent. The nitrogen in nitric acid has an oxidation state of +5, and it can be reduced to lower oxidation states (like +4, +3, +2, or +1), thus it can oxidize other substances.
- Concentrated sulfuric acid is also a strong oxidizing agent. The sulfur in sulfuric acid has an oxidation state of +6 and can be reduced to lower oxidation states. Therefore, it can also act as an oxidizing agent.
- Concentrated hydrochloric acid contains chloride ions (Cl⁻), which are already in their lowest oxidation state (-1). Since chloride cannot be further reduced, concentrated hydrochloric acid does not have the ability to oxidize other substances. Thus, it does not act as an oxidizing agent.
- Based on the analysis, the acid that is not an oxidizing agent is Concentrated Hydrochloric Acid (Conc HCl).
The acid which is not an oxidizing agent is Concentrated Hydrochloric Acid (Conc HCl).
टॉपर्स ने हल किए ये सवाल
- STUDY OF COMPOUNDS - HYDROGEN CHLORIDEBOOK - ICSECHAPTER - STUDY OF COMPOUNDS - HYDROGEN CHLORIDEEXERCISE - ADDITIONAL QUESTIONS
27 Videos
- STUDY OF COMPOUNDS - AMMONIA BOOK - ICSECHAPTER - STUDY OF COMPOUNDS - AMMONIA EXERCISE - UNIT TEST PAPER 7B - AMMONIA
22 Videos
- STUDY OF COMPOUNDS - NITRIC ACID BOOK - ICSECHAPTER - STUDY OF COMPOUNDS - NITRIC ACID EXERCISE - UNIT TEST PAPER 7 C - NITRIC ACID
27 Videos
समान प्रश्न
Give reason - Hydrogen chloride can be termed as a polar covalent compound.
View SolutionChoose the letter corresponding to the correct answer from - A: NO2,B:NO,C:N2,D:N2O. The gas obtained when - Dry ammonia and dry oxygen gas are ingnited together.
View SolutionSelect the letters A,B,C,D or E , which form the gaseous products of the reactions from 1 to 5.
A : nitrogen dioxide only B: Nitric oxide only C : Hydrogen D : Nitrogen dioxide & oxygen E, Nitrogen dioxide & carbon dioxide
Reaction of manganese with cold very dil nitric acid .
View SolutionMixture of conc. HNO_(3) and conc. H_(2)SO_(4) is known as
View SolutionAnisole with conc. HNO3 and conc. H2SO4 gives
View SolutionPheol reacts with conc. HNO3 in the presence of conc. H2SO4 to give :
View Solution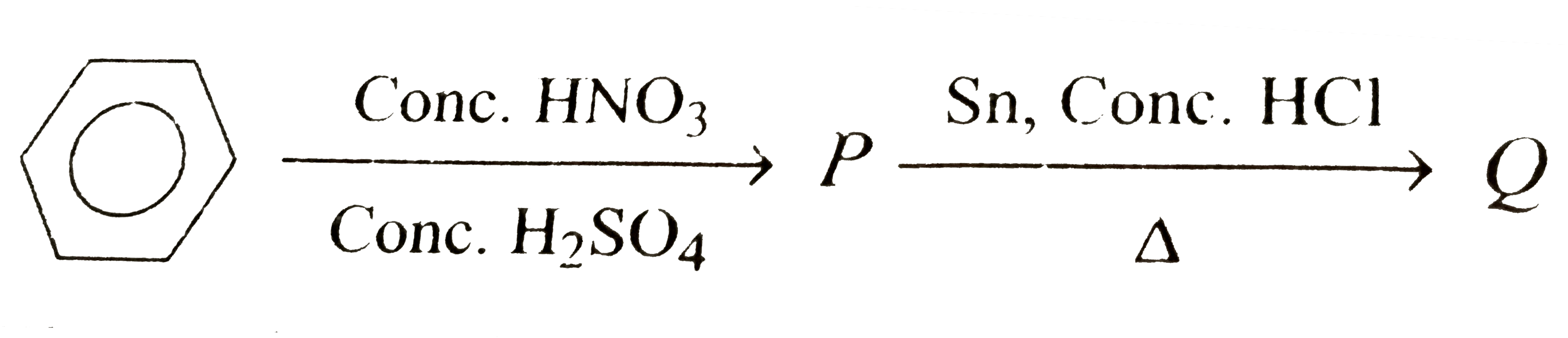 underset (Conc. H_(2)SO_(4))overset(Conc. HNO_(3))toP underset(Delta)overset(Sn, Conc. HCl)toQ
underset (Conc. H_(2)SO_(4))overset(Conc. HNO_(3))toP underset(Delta)overset(Sn, Conc. HCl)toQ
The product Q is :
View SolutionIn a nitrating mixture (conc. HNO_(3)+ conc. H_(2)SO_(4) ) nitric acid acts as
View SolutionNitration of benzene is carried out with conc. HNO_(3) in presence of conc. H_(2)SO_(4) . The role of conc. H_(2)SO_(4) is to provide
View SolutionConc. HNO_(3)+H_(2)SO_(4) mixture gives
View SolutionPicric acid is obtained by heating phenol in the presence of conc. H2SO4 with conc. HNO3/conc.HNO2
View SolutionReaction of anisole with conc. HNO_(3) and conc. H_(2)SO_(4) will give…..
View Solution(A): Conc.H2SO4 reacts with KCl to give Cl2 gas
(R): HCI cannot be oxidised by conc.H2SO4
View SolutionNitration of benzene is carried out with conc. HNO_(3) in presence of conc. H_(2)SO_(4) . The role of conc. H_(2)SO_(4) is to provide
View SolutionSelect the correct word or formula from the same give in bracket :
The substance reacted with conc HCl & heated to prove that conc .HCl containd Cl2.[PbCl2/PbO2/PbO]
View Solution
