The first (IE1) and the second (IE2) ionisation energies (kJ mol−1) of a few elements are given below :
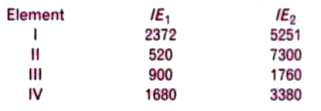
Which of the above elements is likely to be
a noble gas?
Step by step video solution for The first (IE_(1)) and the second (IE_(2)) ionisation energies (kJ mol^(-1)) of a few elements are given below : Which of the above elements is likely to be a noble gas? by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESBook:ICSEChapter:CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESExercise:REVIEW EXERCISES
Explore 29 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESBook:ICSEChapter:CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESExercise:VERY SHORT ANSWER TYPE QUESTIONS
Explore 25 Videos
- CHEMICAL THERMODYNAMICS Book:ICSEChapter:CHEMICAL THERMODYNAMICS Exercise:NCERT TEXT-BOOK. EXERCISES
Explore 22 Videos
- ENVIRONMENTAL CHEMISTRY Book:ICSEChapter:ENVIRONMENTAL CHEMISTRY Exercise:NCERT TEXT-BOOK EXERCISE
Explore 20 Videos
Similar Questions
The first (IE1) and the second (IE2) ionisation energies (kJ mol−1) of a few elements are given below :
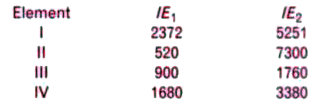
Which of the above elements is likely to be
a reactive non-metal?
View SolutionThe first (IE1) and the second (IE2) ionisation energies (kJ mol−1) of a few elements are given below :
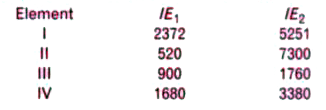
Which of the above elements is likely to be
a reactive metal?
View SolutionThe first (Δi,H1) and the second (Δ,H2) ionisation enthalpies (in kJ mol−1 ) and the (ΔegH) electron gain enthalpy (in kJ mol−1 ) of a few elements are given below :
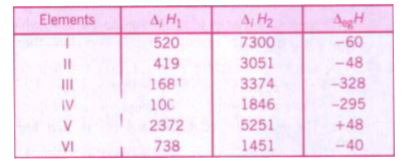
Which of the above elements is likely to be :
the least reactive element?
View SolutionThe first (Δi,H1) and the second (Δ,H2) ionisation enthalpies (in kJ mol−1 ) and the (ΔegH) electron gain enthalpy (in kJ mol−1 ) of a few elements are given below :
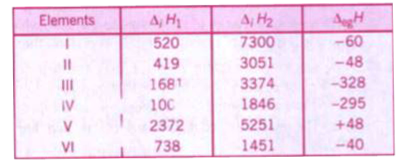
Which of the above elements is likely to be :
a reactive metal
View SolutionThe first (IE1) and second (E2) ionization energies (kJ/mol) of few elements designated by Roman numerals are given below. Which of these would be an alkali metal?
View SolutionFirst and second ionisation enthalpies(in KJ/mol) of few elements are given below:
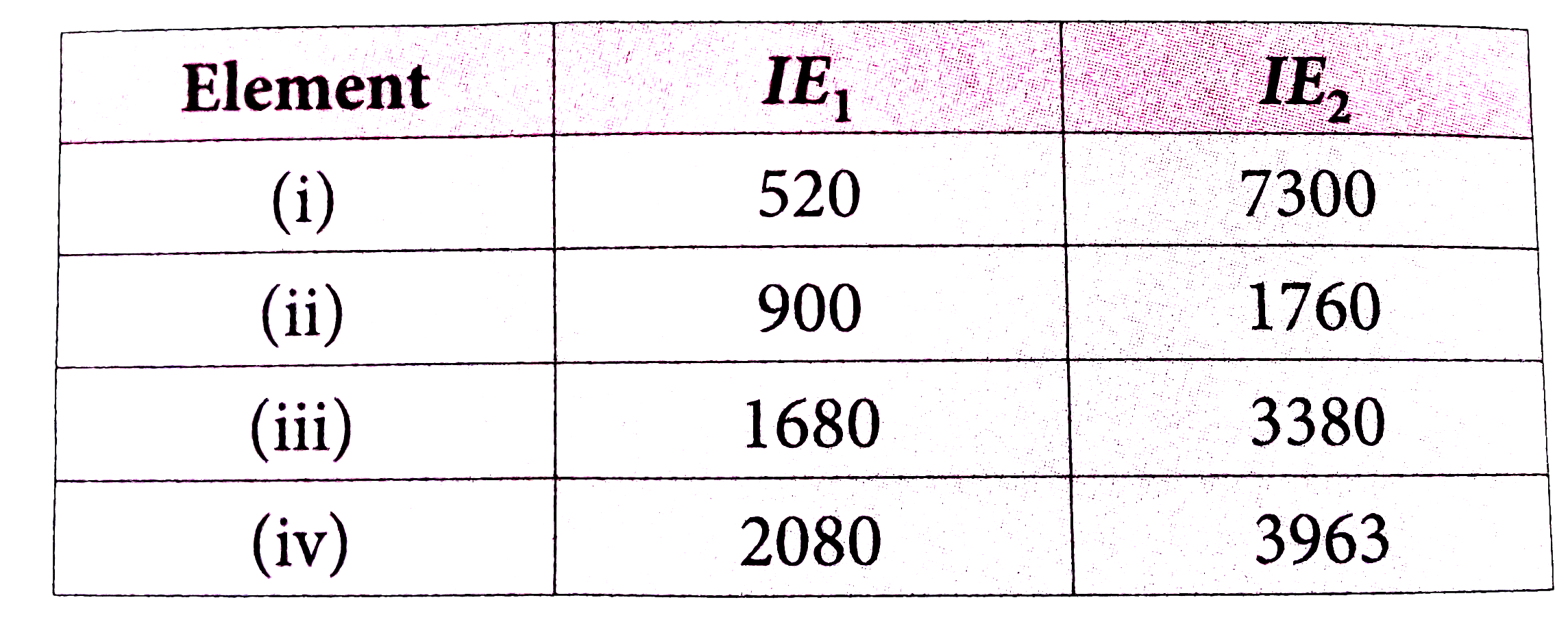
Which of the above elements will form halides with formula MX2?
View SolutionIonization energies of five elements in kcal/mol are given below :
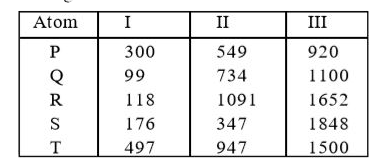
Which element is a noble gas ?
View SolutionIonization energies of five elements in kcal/mol are given below:
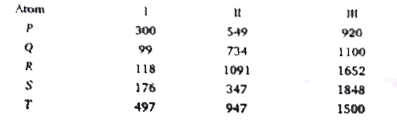
Q. Which element is a noble gas?
View SolutionIonization energies of five elements in kcal/mol are given below:
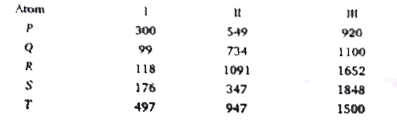
Q. Which element is a noble gas?
View SolutionIonization energies of five elements in kcal/mol are given below:
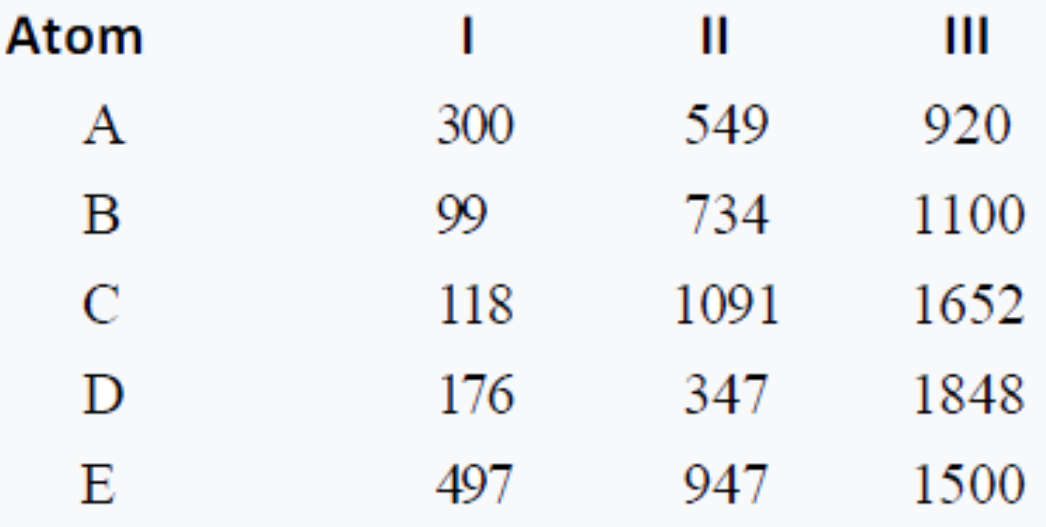 .
.
Which element is a noble gas ?
View Solution
