Show that the entropy change in an irreversible process is always positive.
Step by step video solution for Show that the entropy change in an irreversible process is always positive. by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CHEMICAL THERMODYNAMICS Book:ICSEChapter:CHEMICAL THERMODYNAMICS Exercise:ESSAY (LONG ANSWER) TYPE QUESTIONS
Explore 32 Videos
- CHEMICAL THERMODYNAMICS Book:ICSEChapter:CHEMICAL THERMODYNAMICS Exercise:OBJECTIVE (MULTIPLE CHOCE) TYPE QUESTIONS
Explore 59 Videos
- CHEMICAL THERMODYNAMICS Book:ICSEChapter:CHEMICAL THERMODYNAMICS Exercise:VERY SHORT ANSWER TYPE QUESTIONS
Explore 56 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:ICSEChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:NCERT TEXT-BOOK EXERCISES ( WITH HINTS AND SOLUTIONS)
Explore 47 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESBook:ICSEChapter:CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESExercise:NCERT TEXT-BOOK. EXERCISE (With Hints and Solutions)
Explore 63 Videos
Similar Questions
Which is an irreversible process ?
View SolutionShow that the entropy changes in a reversible isothermal process is zero.
View SolutionEntropy change for an adiabatic reversible process is
View SolutionFor an irreversible process, ΔS>0.
View SolutionWhich of the following are irreversible processes
View SolutionWhich of the following expression for entropy change of an irreversible process ?
View SolutionCalculate the total entropy change for the following reversible processes:
a. Isothermal b. Adiabatic
View SolutionFind work done in the irreversible process C→A.
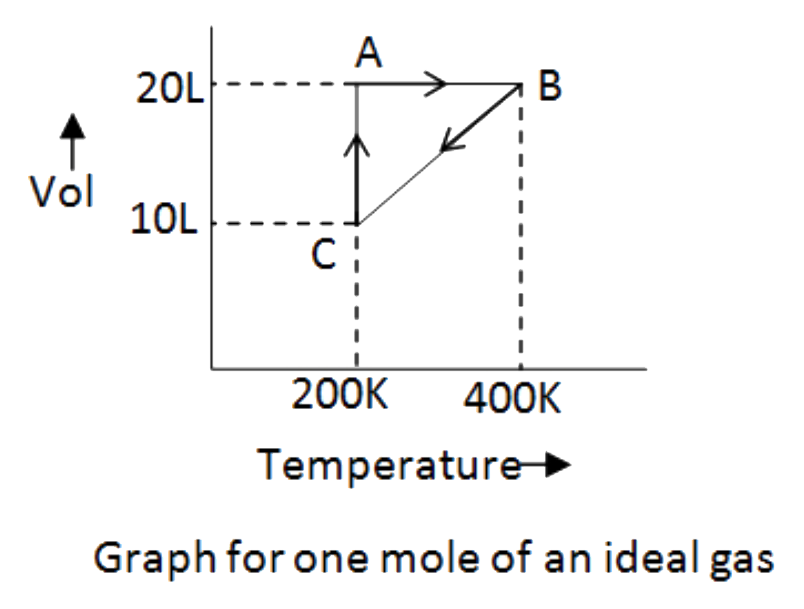
View SolutionGrowth is irreversible process found at organisation level
View SolutionEntropy (S) is a thermodynamic variable like pressure P, volume V and temperature T. Entropy of a thermodynamic system is a measure of disorder of molecular motion. Greater is disorder , greater is entropy. Change in entropy of a thermodynamic system is the ratio of heat supplied to absolute temperature.In an adiabatic reversible process, entropy remains constant while in any irreversible process entropy increases. In nature the processes are irreversible , therefore entropy of universe is continuoulsy increasing.
The unit of entropy in S−I system is -
View Solution
