When the work is done by the system, w is taken as ......
To solve the question, we need to understand the convention used in thermodynamics regarding work done by or on a system.
1. Understanding Work in Thermodynamics: In thermodynamics, work can be done by a system or on a system. This is a crucial concept as it affects the energy balance of the system.
2. IUPAC Convention: According to the International Union of Pure and Applied Chemistry (IUPAC) conventions:
- When work is done by the system (for example, when a gas expands and pushes against the surroundings), it is considered as energy leaving the system.
- Conversely, when work is done on the system (for example, when a gas is compressed), it is considered as energy entering the system.
3. Sign Convention:
- If the system does work on the surroundings, we denote this work as negative (w < 0).
- If work is done on the system, we denote this work as positive (w > 0).
4. Conclusion: Therefore, when the work is done by the system, we take w as negative.
Final Answer:
When the work is done by the system, w is taken as negative.
- When work is done by the system (for example, when a gas expands and pushes against the surroundings), it is considered as energy leaving the system.
- Conversely, when work is done on the system (for example, when a gas is compressed), it is considered as energy entering the system.
- If the system does work on the surroundings, we denote this work as negative (w < 0).
- If work is done on the system, we denote this work as positive (w > 0).
When the work is done by the system, w is taken as negative.
Topper's Solved these Questions
- CHEMICAL THERMODYNAMICS Book:ICSEChapter:CHEMICAL THERMODYNAMICS Exercise:ASSERTION -REASON TYPE QUESTIONS
Explore 9 Videos
- CHEMICAL THERMODYNAMICS Book:ICSEChapter:CHEMICAL THERMODYNAMICS Exercise:NUMBERICAL PROBLEMS
Explore 18 Videos
- CHEMICAL THERMODYNAMICS Book:ICSEChapter:CHEMICAL THERMODYNAMICS Exercise:TRUE OR FALSE. TYPE QUESTIONS
Explore 24 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:ICSEChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:NCERT TEXT-BOOK EXERCISES ( WITH HINTS AND SOLUTIONS)
Explore 47 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESBook:ICSEChapter:CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESExercise:NCERT TEXT-BOOK. EXERCISE (With Hints and Solutions)
Explore 63 Videos
Similar Questions
if work is done by the system then W is
View SolutionWhat is the value of ΔE when the work done by the system is equal to the heat absorbed by the system?
View SolutionWe consider a thermodynamic system. If ΔU represents the increase in its internal energy and W the work done by the system, which of the following statements is true?
View SolutionIf W is the amount of work done by the system and q is the amount of heat supplied to the system, identify the type of the system
View SolutionIn given figure, let ΔW and ΔW2 be the work done by the gas in process A and B respectively then (given change in volume is same in both process)
View SolutionIn a cyclic process, work done by the system is
View SolutionWhen a system is taken from state a to state b, in figure along the path a→c→b, 60J of heat flow into the system, and 30J of work is done :
(i) How much heat flows into the system along the path a→d→b if the work is 10J.
(ii) When the system is returned from b to along the curved path, the work done by the system is −20J. Does the system absorb or liberate heat, and how much ?
(iii) If , Ua=0 and Ud=22J, find the heat absorbed in the process a→d and d→b.
_S01_052_Q01.png)
View SolutionPick out the correct option which represents for the work done by the system on the surroundings
View SolutionIn given figure, let ΔU1 and ΔU2 be change in internal energy in process ABC and CDA respectively. ΔQ and W be the net heta given and net work done by the system in the process ABC+CDA, then
View SolutionWhen a system is taken from A to C through path ABC, 10 J of heat flows to the system and 4 J of work is done by the system.
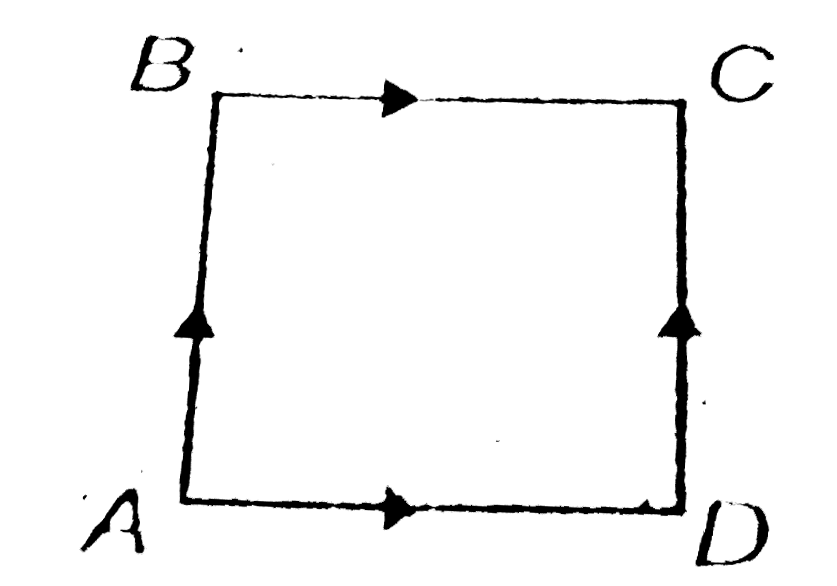
How much heat flows into the system in path ADC, if the work done by the system is 3 J ?
View Solution
