Draw the orbital diagram of 40 20Ca2+ ion and state the number of three fundamental particles present in it.
To solve the question, we will follow these steps:
Step 1: Determine the number of electrons in Ca²⁺
- Calcium (Ca) has an atomic number of 20, which means it has 20 protons and, in its neutral state, 20 electrons.
- Since Ca²⁺ indicates that the calcium atom has lost 2 electrons, the number of electrons in Ca²⁺ is:
\(
20 - 2 = 18 \text{ electrons}
\)
Step 2: Write the electronic configuration of Ca²⁺
- The electronic configuration of a neutral calcium atom (Ca) is:
\(
1s^2 \, 2s^2 \, 2p^6 \, 3s^2
\)
- For Ca²⁺, we remove 2 electrons from the outermost shell (3s), resulting in:
\(
1s^2 \, 2s^2 \, 2p^6 \, 3s^0
\)
- This can be simplified to:
\(
1s^2 \, 2s^2 \, 2p^6 \quad \text{or} \quad (Ne) \text{ (Neon configuration)}
\)
- Thus, the final configuration is:
\(
2, 8, 8
\)
Step 3: Draw the orbital diagram for Ca²⁺
- The orbital diagram represents the arrangement of electrons in the shells:
- K shell (1st shell): 2 electrons
- L shell (2nd shell): 8 electrons
- M shell (3rd shell): 8 electrons
The orbital diagram can be represented as follows:
K↑↓L↑↓↑↓↑↓↑↓↑↓↑↓↑↓↑↓M↑↓↑↓↑↓↑↓↑↓↑↓↑↓↑↓
Step 4: Count the fundamental particles in Ca²⁺
- Electrons: 18 (as calculated)
- Protons: The atomic number of calcium is 20, so there are 20 protons.
- Neutrons: The mass number of calcium is 40. The number of neutrons can be calculated as:
\(
\text{Neutrons} = \text{Mass number} - \text{Protons} = 40 - 20 = 20
\)
Summary of Fundamental Particles
- Electrons: 18
- Protons: 20
- Neutrons: 20
Final Answer
The orbital diagram of Ca²⁺ is shown above, and the number of fundamental particles present in it are:
- Electrons: 18
- Protons: 20
- Neutrons: 20
- Calcium (Ca) has an atomic number of 20, which means it has 20 protons and, in its neutral state, 20 electrons.
- Since Ca²⁺ indicates that the calcium atom has lost 2 electrons, the number of electrons in Ca²⁺ is:
\(
20 - 2 = 18 \text{ electrons}
\)
- The electronic configuration of a neutral calcium atom (Ca) is:
\(
1s^2 \, 2s^2 \, 2p^6 \, 3s^2
\)
- For Ca²⁺, we remove 2 electrons from the outermost shell (3s), resulting in:
\(
1s^2 \, 2s^2 \, 2p^6 \, 3s^0
\)
- This can be simplified to:
\(
1s^2 \, 2s^2 \, 2p^6 \quad \text{or} \quad (Ne) \text{ (Neon configuration)}
\)
- Thus, the final configuration is:
\(
2, 8, 8
\)
- The orbital diagram represents the arrangement of electrons in the shells:
- K shell (1st shell): 2 electrons
- L shell (2nd shell): 8 electrons
- M shell (3rd shell): 8 electrons
- Electrons: 18 (as calculated)
- Protons: The atomic number of calcium is 20, so there are 20 protons.
- Neutrons: The mass number of calcium is 40. The number of neutrons can be calculated as:
\(
\text{Neutrons} = \text{Mass number} - \text{Protons} = 40 - 20 = 20
\)
- Electrons: 18
- Protons: 20
- Neutrons: 20
The orbital diagram of Ca²⁺ is shown above, and the number of fundamental particles present in it are:
- Electrons: 18
- Protons: 20
- Neutrons: 20
Topper's Solved these Questions
- ATOMIC STRUCTURE AND CHEMICAL BONDINGBook:ICSEChapter:ATOMIC STRUCTURE AND CHEMICAL BONDINGExercise:EXERCISE 4(C)
Explore 54 Videos
- ATOMIC STRUCTURE AND CHEMICAL BONDINGBook:ICSEChapter:ATOMIC STRUCTURE AND CHEMICAL BONDINGExercise:EXERCISE 4(D)
Explore 21 Videos
- ATOMIC STRUCTURE AND CHEMICAL BONDINGBook:ICSEChapter:ATOMIC STRUCTURE AND CHEMICAL BONDINGExercise:EXERCISE 4(D)
Explore 21 Videos
- ATMOSPHERIC POLLUTIONBook:ICSEChapter:ATMOSPHERIC POLLUTIONExercise:EXERCISE 8(D)
Explore 38 Videos
- CHEMICAL CHANGES AND REACTIONSBook:ICSEChapter:CHEMICAL CHANGES AND REACTIONSExercise:EXERCISE 2(C)
Explore 30 Videos
Similar Questions
From the symbol 24 12Mg, state the mass number, the atomic number and electronic configuration of magnesium.
View SolutionHow many number of unpaired electrons are present in Ca2+ ?
View Solution4.0 g of caustic soda (NaOH) contains same number of sodium ions as are present in :
View SolutionDraw the energy level diagram of H+2 ion and discuss its stability in terms of bond order.
View Solution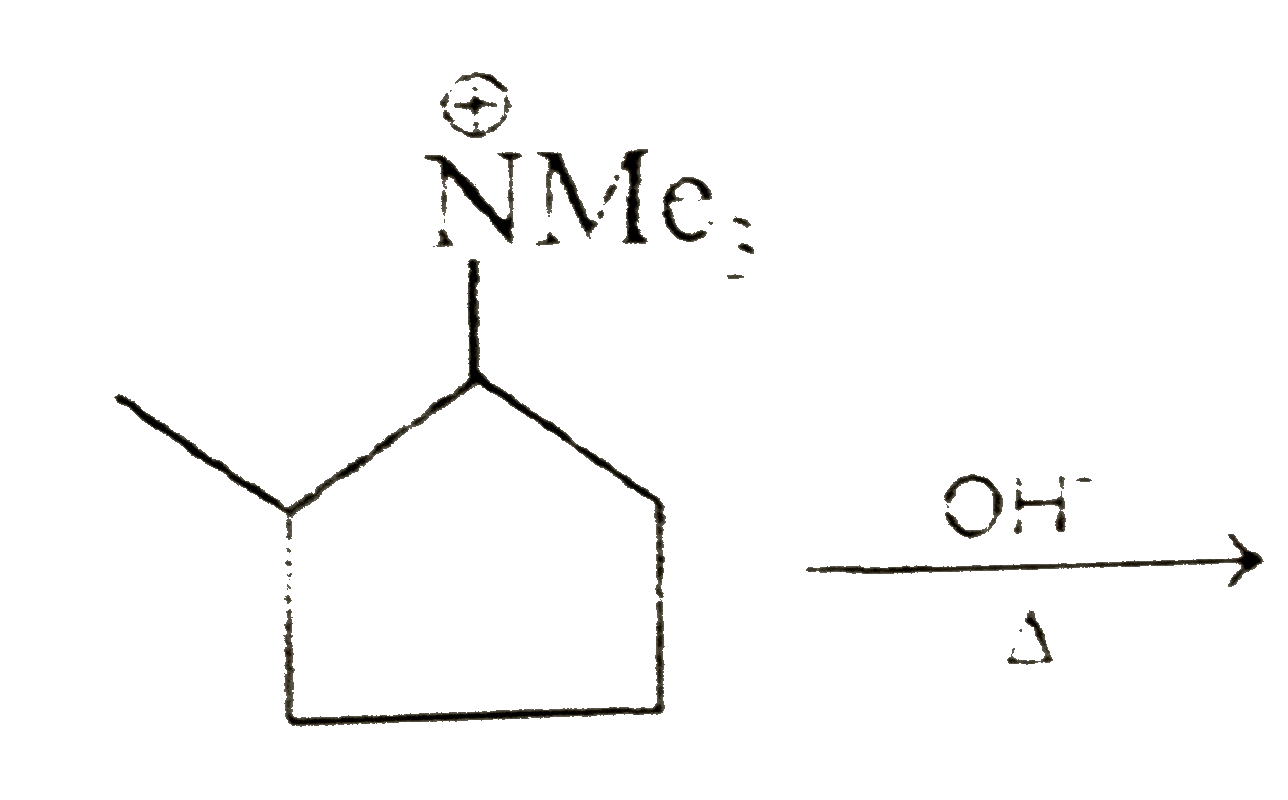 OH−−−→Δ number of hyperconjugate hydrogen atoms present in major product.
OH−−−→Δ number of hyperconjugate hydrogen atoms present in major product.
View SolutionDraw the molecular orbital diagrams of H2,H+2andH−2 and discuss their relative stabilities.
View SolutionDraw a neat labeled diagram representing an atom. Name the three sub-atomic particles in the atom & represent them symbolically showing the mass & charge of each. State where the sub-atomic particles are present in the atom.
View SolutionHexafluorocobaltate (III) ion is an outer orbital complex. The
number of unpaired electrons present in it is
View SolutionDraw the molecular orbital diagram of O2 molecule. How would you explain the paramagnetic nature of the molecule on the basis of this diagram?
View SolutionThe total number of covalent bonds present in the NO+2 ion is :
View Solution