Write balanced chemical equations for the following:
A mixture of soda lime and sodium acetate is heated.
CH3CH2COONa+NaOHCaO−−→C2H6+Na2CO3
Topper's Solved these Questions
- SAMPLE QUESTION PAPER 02Book:ICSEChapter:SAMPLE QUESTION PAPER 02Exercise:SECTION ll
Explore 44 Videos
- SAMPLE QUESTION PAPER -4Book:ICSEChapter:SAMPLE QUESTION PAPER -4Exercise:SECTION - II
Explore 85 Videos
- SAMPLE QUESTION PAPER 03Book:ICSEChapter:SAMPLE QUESTION PAPER 03Exercise:SECTION II
Explore 47 Videos
Similar Questions
Show the formation ofH3O+ using the electron dot diagram.
View SolutionSome properties of sulphuric acid are listed below. Choose the property A, B, C or D which is responsible for the reactions (i) to (v). Some properties may be repeated :
C12H22O11+nH2SO4→12C+11H2O+nH2SO4
View SolutionThe following question relate to the extraction of aluminium by electrolysis:
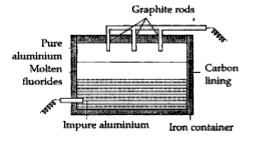
Name the other aluminium containing compound added to alumina and state its significance.
View SolutionHeating a mixture of sodium benzoate and soda-lime gives
View SolutionAnhydrous sodium acetate on heating with soda lime gives _______.
View SolutionHeating mixture of sodium benzoate and soda-lime gives
View SolutionHeating a mixture of sodium acetate and soda lime gives
View SolutionHeating a mixture of sodium acetate and soda lime gives
View SolutionHeating a mixture of sodium acetate and soda lime gives
View SolutionHeating a mixture of sodium acetate and soda lime gives
View SolutionHeating a mixture of sodium acetate and soda lime gives
View SolutionAnhydrous sodium acetate on heating with soda lime gives _______.
View SolutionSodium acetate is formed when heated with soda lime.
View SolutionSodium acetate on heating with soda lime produce
View SolutionWhat happens when (give only balanced equations and write the names of the reactants and products ) A mixture of sodium acetate and soda lime is heated.
View SolutionWrite balanced chemical equations for the following: A mixture of soda lime and sodium acetate is heated.
View Solution