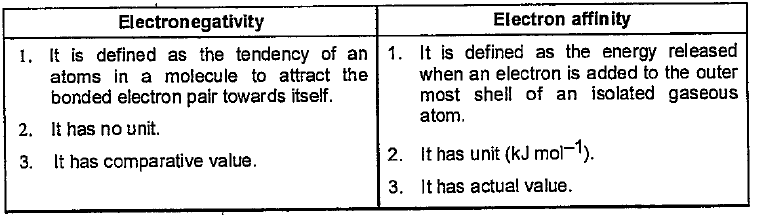
Write differences between electronegativity and electron affinity.Solution in Bengali
Topper's Solved these Questions
Similar Questions
AlCl3 forms a dimer but BCl3 does not form dimer.Explain.
View SolutionZn(s)+Cu2+→Zn2++Cu(s) in this reaction which is oxidant and which is reductant?
View SolutionIf x,y and z are electronegativity, ionisation potential and electron−affinity respectively. Then the electron affinity (z) in the terms of electronegativity (x) and ionisation potential (y) will be :
View SolutionIf x,y and z are Mulliken electronegativity, ionisation potential and electron-affinity respectively. Then the electron affinity (z) in the terms of electronegativity (x) and ionisation potential (y) will be:
View SolutionPERIODICITY IN ELECTRON AFFINITY , ELECTRONEGATIVITY
View Solutionइलेक्ट्रॉन बन्धुता तथा विद्युत् ऋणात्मकता में क्या अन्तर है ?
View SolutionGive the definition of electron affinity and electronegativity. Write their differences.
View SolutionWhat is the difference between ionisation potential and electron affinity?
View Solutionकिसी तत्व की विघुत ऋणात्मक से क्या समझते हैं ? इलेक्ट्रॉन बंधुता से यह किस प्रकार भिन्न हैं ? आवर्त सारणी में विघुत ऋणात्मकता किस प्रकार बदलती हैं ?
View Solutionఎలక్ట్రాన్ అఫినిటీ, ఋణ విద్యుదాత్మకతల తేడా ఏమిటి?
View SolutionThe elements with the highest electron affinity and electronegativity respectively are
View SolutionElectronegativity of an element is the average of its ionisation energy and electron affinity according to
View SolutionThe elements with the highest electron affinity and electronegativity respectively are
View SolutionWrite the differences between electron affinity and electronegativity.
View SolutionGive differences between electron affinity and electronegativity.
View Solution