The magnetic moment for two complexes of empirical formula Ni(NH3)4(NO3)2⋅2H2O is zero and 2.84 BM respectively. The second complex is not a neutral complex.
Q. The correct formula and geometry of the first complex is:
[Ni(H2O)2(NO3)2]⋅4NH3, tetrahedral.
[Ni(NH3)4](NO3)2⋅2H2O,tetrahedral.
[Ni(NH3)4](NO3)2⋅2H2O,square planar
[Ni(NH3)4(H2O)2](NO3)2,octahedral.
The correct Answer is:C
Similar Questions
[(NH3)5Co−O−O−Co(NH3)5]4+[S2O8]2−−−−−−→oxidise[(NH3)5Co−O−O−Co(NH3)5]5+ (Brown) (Green)
The magnetic moment of green complex is 1.7 BM and for brown complexes magnetic moment is zero. (O−O) is same in all respect in both the complexes. The oxidation state of Co in brown complex and green complex respectively are :
View SolutionMn^(2+) forms a complex with Br– ion. The magnetic moment of the complex is 5.92 B. M. What could not be the probable formula and geometry of the complex?
View Solution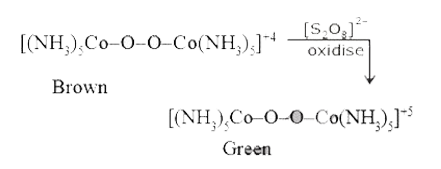
The magnetic moment of green complex is 1.7 BM & for brown complexes magnetic moment is zero. (O–O) is same in all respect in both the complexes. The O. S. of Co in brown complex & green complex respectively are–
View SolutionThe geometry and magnetic behaviour of the complex [Ni(CO)_(4)] are -
View SolutionThe geometry and magnetic behaviour of the complex [Ni(CO)4] are-
View SolutionMn2+ forms a complex with Br– ion. The magnetic moment of the complex is 5.92 B. M. What could not be the probable formula and geometry of the complex?
View SolutionThe magnetic moment for two complexes of empirical formula Ni(NH_(3))_(4)(NO_(3))_(2)*2H_(2)O is zero and 2.84 BM respectively. The second complex is not a neutral complex. Q. Which of the following statements are true for the second complex ?
View Solution

