Calculate the order of the reaction A→ product from the following data :
 .
.
Video Solution
Text Solution
Let the order be n .
Applying rate law for the first and second set of readings ,
we have
rate = 0×10−5=k(0.0025)n …(1)
and , rate = 5×10−5=k(0.005)5 …(2)
Dividing (1) by (2) , we get 2=(12)th
orn=−1
Thus the order of the reaction is = - 1
|
Topper's Solved these Questions
- CHEMICAL KINETICSBook:RC MUKHERJEEChapter:CHEMICAL KINETICSExercise:PROBLEMS
Explore 42 Videos
- CHEMICAL KINETICSBook:RC MUKHERJEEChapter:CHEMICAL KINETICSExercise:OBJECTIVE PROBLEMS
Explore 51 Videos
- CHEMICAL EQUIVALENCEBook:RC MUKHERJEEChapter:CHEMICAL EQUIVALENCEExercise:PROBLEMS
Explore 24 Videos
- CHEMICAL THERMODYNAMICSBook:RC MUKHERJEEChapter:CHEMICAL THERMODYNAMICSExercise:Objective problems
Explore 52 Videos
Similar Questions
Knowledge Check
Calculate the order of reaction, A → Product, from the following data:
[A]d[Product]/dt(moles/L)(moles/L/s)0.00310.0×10−50.0065.0×10−50.0122.5×10−5A1B−2C−1D2
Calculate value of output from the following data :

View SolutionCalculate value of output from the following data :
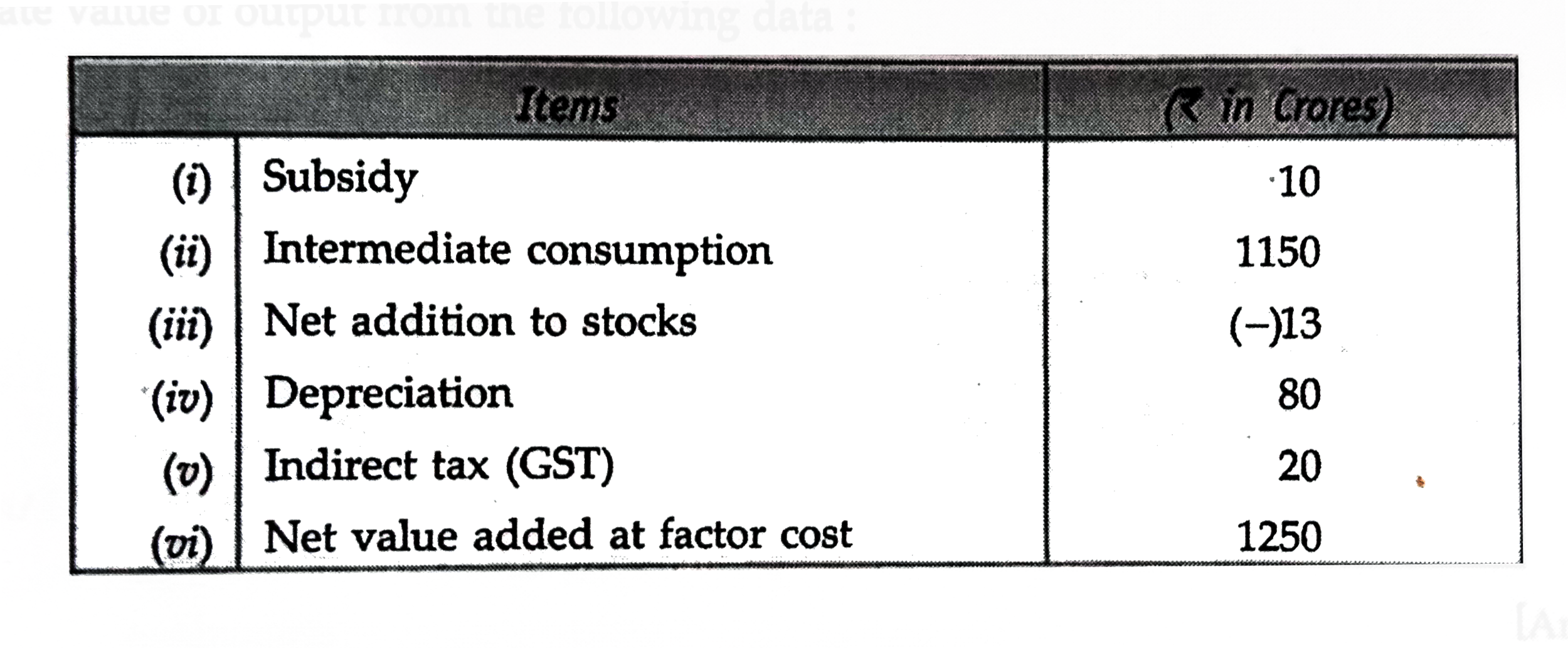
View SolutionCalculate value of output from the following data :

View SolutionCalculate the order of reaction A+B→P, with respect to A from the following data :

View SolutionCalculate median from the following data :
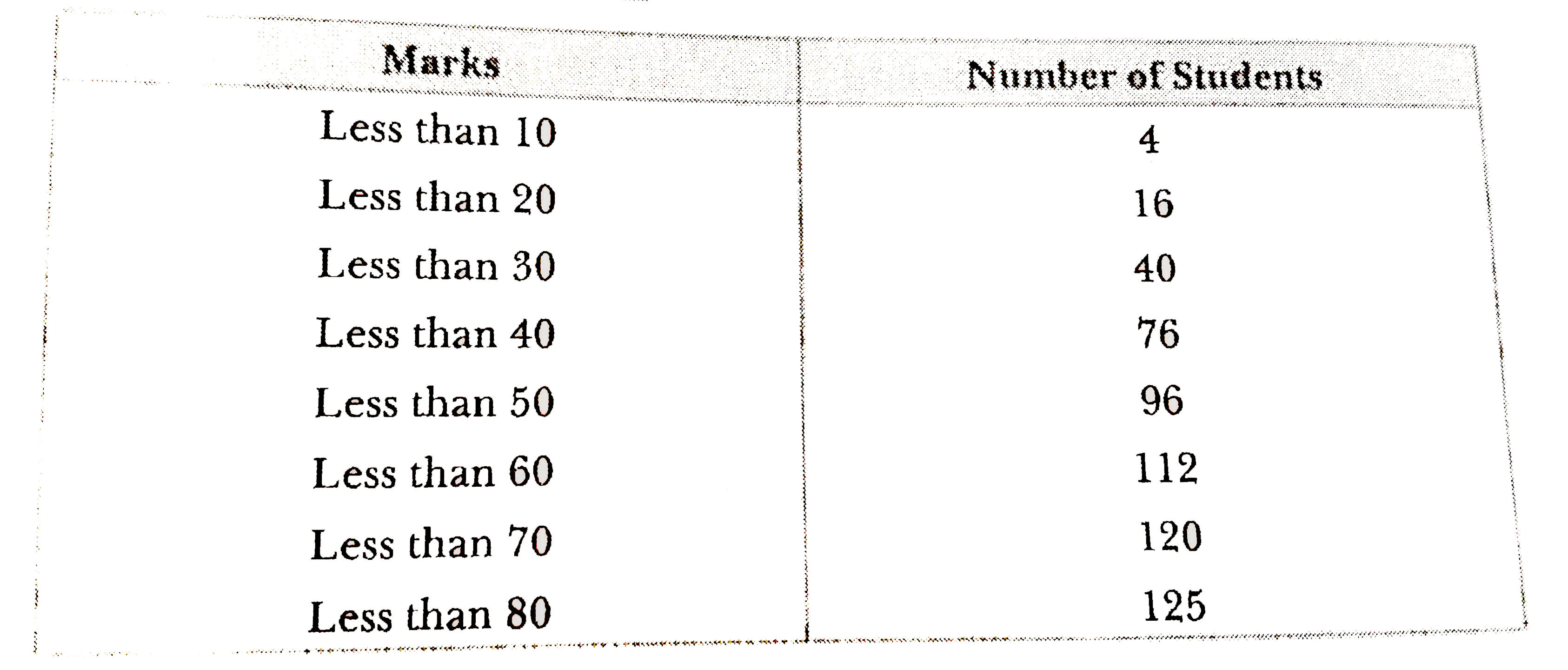
View SolutionCalculate National income and from the following data :
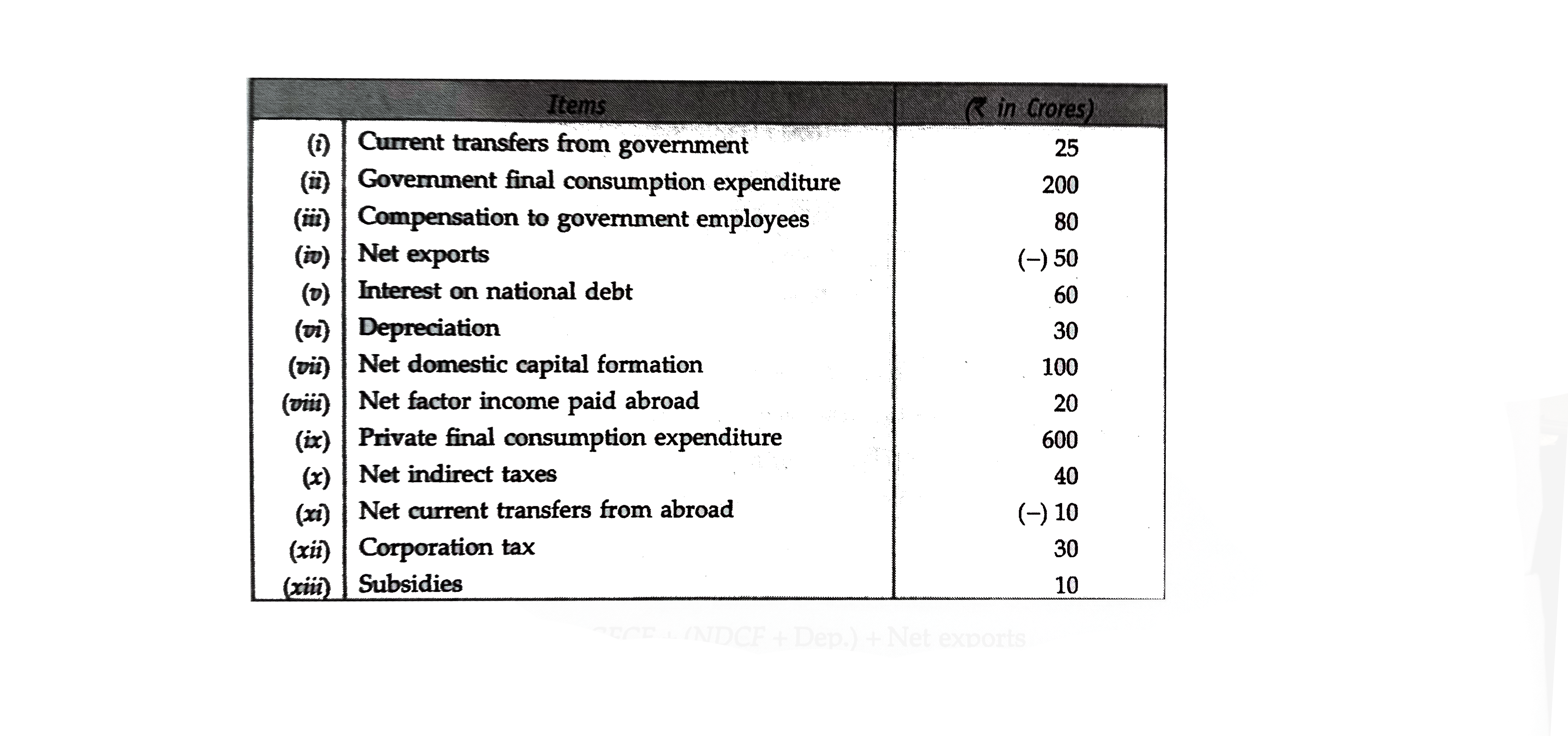
View SolutionCalculate Sales from the following data :

View Solution