When chlorine is passed over dry slaked lime at room tempreture, the main reaction product is
Ca(ClO2)2
CaCl2
CaOCl2
Ca(OCl)2
The correct Answer is:C
To determine the main reaction product when chlorine is passed over dry slaked lime at room temperature, we can follow these steps:
Step 1: Identify the reactants
- The reactants in this reaction are chlorine gas (Cl₂) and dry slaked lime, which is chemically represented as calcium hydroxide (Ca(OH)₂).
Step 2: Understand the reaction conditions
- The reaction takes place at room temperature, which is important because it influences the products formed.
Step 3: Write the chemical equation
- When chlorine gas reacts with dry slaked lime, the main product formed is bleaching powder, which has the chemical formula Ca(OCl)₂ (calcium oxychloride). The reaction can be represented as:
Ca(OH)2+Cl2→Ca(OCl)2+H2O
Step 4: Identify the main product
- The main product of this reaction is bleaching powder (Ca(OCl)₂).
Conclusion
- Therefore, when chlorine is passed over dry slaked lime at room temperature, the main reaction product is bleaching powder (Ca(OCl)₂).
---
- The reactants in this reaction are chlorine gas (Cl₂) and dry slaked lime, which is chemically represented as calcium hydroxide (Ca(OH)₂).
- The reaction takes place at room temperature, which is important because it influences the products formed.
- When chlorine gas reacts with dry slaked lime, the main product formed is bleaching powder, which has the chemical formula Ca(OCl)₂ (calcium oxychloride). The reaction can be represented as:
Ca(OH)2+Cl2→Ca(OCl)2+H2O
- The main product of this reaction is bleaching powder (Ca(OCl)₂).
- Therefore, when chlorine is passed over dry slaked lime at room temperature, the main reaction product is bleaching powder (Ca(OCl)₂).
समान प्रश्न
ज्ञान जांच
In the given reaction the main product will be:
 A
A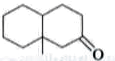 B
B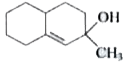 C
C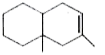 D
D
In the given reaction the main product will be:
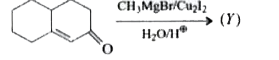 A
A B
B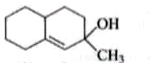 C
C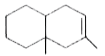 D
D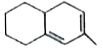
A balck powder when heated with Conc. HCI gives a greenish yellow. Gas. The gas as an oxidising and bleaching agent. When it is passed over slake lime, a white poweder is formed which is a ready source of gas. The back powder and white powder respectively are
AKClO3andNaClO3BMnO2andCa(OCl)2CMnO2andKClO3DMnCl4andCOCl2
What happens when steam is passed over magnesium?
View SolutionState a reason why when hydrogen is passed over heated copper oxide, the resultant product fromed, differs incolour from the original reactant.
View SolutionWhen dry ammonia gas is passed over heated sodium (out of contact of air) the product forms is
View SolutionWhen tertiary butyl alcohol is passed over reduced copper, the reaction taking placed is
View SolutionWhen tertiary butyl alcohol is passed over reduced copper, the reaction taking placed is
View Solution

