Which one of the following represent nylon 6,6 polymer?
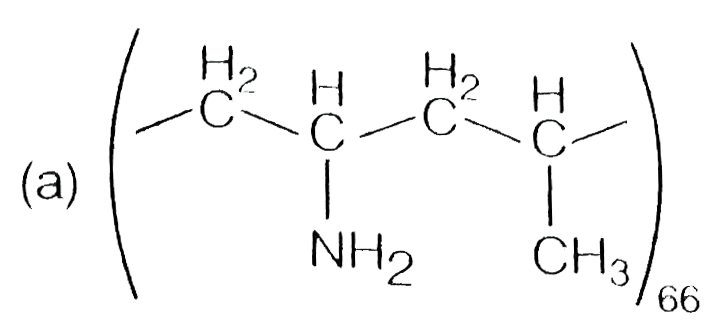
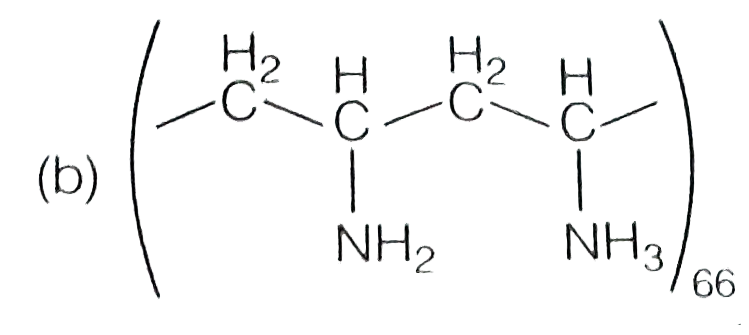
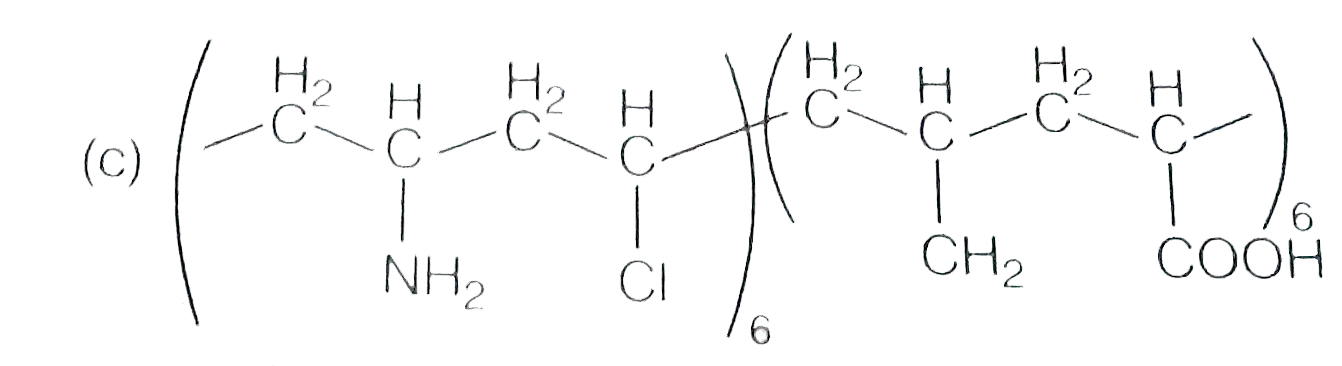
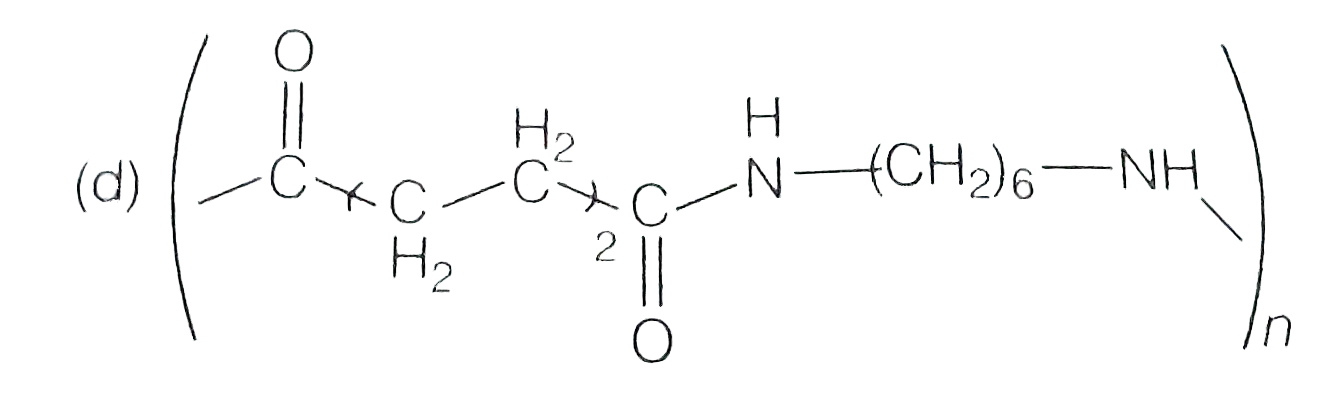
The correct Answer is:D
To determine which structure represents the Nylon-6,6 polymer, we need to understand its composition and formation. Here's a step-by-step solution:
Step 1: Identify the Monomers
Nylon-6,6 is a type of polyamide formed from two specific monomers:
1. Adipic Acid: This is a dicarboxylic acid with the formula HOOC-(CH2)4-COOH.
2. Hexamethylenediamine: This is a diamine with the formula H2N-(CH2)6-NH2.
Step 2: Understand the Polymerization Process
Nylon-6,6 is synthesized through a condensation polymerization reaction between the two monomers. In this process:
- The carboxylic acid group (-COOH) from adipic acid reacts with the amine group (-NH2) from hexamethylenediamine.
- This reaction results in the formation of an amide bond (-CO-NH-) and releases a molecule of water (H2O).
Step 3: Write the Structure of Nylon-6,6
The repeating unit of Nylon-6,6 can be represented as follows:
- The backbone consists of alternating carbon and nitrogen atoms due to the amide linkages.
- The structure can be depicted as:
- COO-(CH2)4-CO-NH-(CH2)6-NH
Step 4: Identify the Correct Representation
From the options provided in the question, we need to look for the structure that reflects the repeating unit of Nylon-6,6, which includes:
- The amide linkages
- The correct number of carbon atoms (6 from each monomer)
Conclusion
The correct representation of the Nylon-6,6 polymer is the structure that shows the alternating amide linkages and the correct number of carbon atoms from both monomers.
Final Answer
The correct option that represents the Nylon-6,6 polymer is Option D.
---
Nylon-6,6 is a type of polyamide formed from two specific monomers:
1. Adipic Acid: This is a dicarboxylic acid with the formula HOOC-(CH2)4-COOH.
2. Hexamethylenediamine: This is a diamine with the formula H2N-(CH2)6-NH2.
Nylon-6,6 is synthesized through a condensation polymerization reaction between the two monomers. In this process:
- The carboxylic acid group (-COOH) from adipic acid reacts with the amine group (-NH2) from hexamethylenediamine.
- This reaction results in the formation of an amide bond (-CO-NH-) and releases a molecule of water (H2O).
The repeating unit of Nylon-6,6 can be represented as follows:
- The backbone consists of alternating carbon and nitrogen atoms due to the amide linkages.
- The structure can be depicted as:
- COO-(CH2)4-CO-NH-(CH2)6-NH
From the options provided in the question, we need to look for the structure that reflects the repeating unit of Nylon-6,6, which includes:
- The amide linkages
- The correct number of carbon atoms (6 from each monomer)
The correct representation of the Nylon-6,6 polymer is the structure that shows the alternating amide linkages and the correct number of carbon atoms from both monomers.
The correct option that represents the Nylon-6,6 polymer is Option D.
Similar Questions
Structures of nylon-6,6 polymer?
View SolutionIllustrate the synthesis of the following polymer with uses. Nylon-6,6
View SolutionWhich one of the following structures represents nylon-6,6 polymer?
View SolutionNylone-6,6 is a polymer of :
View SolutionNylon-6, 6 is _____ polymer.
View SolutionWhich one of the following structures represents nylon 6,6 polymer ?
View SolutionWhich one of the following structures represents nylon-6,6 polymer?
View Solution

