In a typical fuel cell, the reactants(R) and product (P) are:
R=H2(g),O2(g),P=H2O2(l)
R=H2(g),O2(g),P=H2O(l)
R=H2(g),O2(g),Cl2(g),P=HClO4(aq)
R=H2(g),N2(g),P=NH3(aq)
The correct Answer is:B
To solve the question regarding the reactants and products in a typical fuel cell, we can follow these steps:
1. Understanding Fuel Cells:
- A fuel cell is an electrochemical device that converts the chemical energy of a fuel (usually hydrogen) and an oxidant (usually oxygen) into electrical energy.
2. Identifying Reactants:
- In a typical hydrogen fuel cell, the primary reactants are hydrogen (H₂) and oxygen (O₂). These gases are fed into the fuel cell.
3. Chemical Reactions in the Fuel Cell:
- At the anode (the negative electrode), hydrogen undergoes oxidation. This means that hydrogen molecules lose electrons:
2H2→4H++4e−
- At the cathode (the positive electrode), oxygen undergoes reduction. Oxygen molecules gain electrons and react with protons (H⁺) to form water:
O2+4H++4e−→2H2O
4. Overall Reaction:
- The overall reaction in a hydrogen fuel cell can be summarized as:
2H2+O2→2H2O
- This indicates that two molecules of hydrogen react with one molecule of oxygen to produce two molecules of water.
5. Identifying Products:
- The primary product of this reaction is water (H₂O), and heat is also released as a byproduct.
6. Final Answer:
- Therefore, in a typical fuel cell, the reactants are hydrogen and oxygen, and the product is water.
Summary:
- Reactants: Hydrogen (H₂) and Oxygen (O₂)
- Product: Water (H₂O)
- A fuel cell is an electrochemical device that converts the chemical energy of a fuel (usually hydrogen) and an oxidant (usually oxygen) into electrical energy.
- In a typical hydrogen fuel cell, the primary reactants are hydrogen (H₂) and oxygen (O₂). These gases are fed into the fuel cell.
- At the anode (the negative electrode), hydrogen undergoes oxidation. This means that hydrogen molecules lose electrons:
2H2→4H++4e−
- At the cathode (the positive electrode), oxygen undergoes reduction. Oxygen molecules gain electrons and react with protons (H⁺) to form water:
O2+4H++4e−→2H2O
- The overall reaction in a hydrogen fuel cell can be summarized as:
2H2+O2→2H2O
- This indicates that two molecules of hydrogen react with one molecule of oxygen to produce two molecules of water.
- The primary product of this reaction is water (H₂O), and heat is also released as a byproduct.
- Therefore, in a typical fuel cell, the reactants are hydrogen and oxygen, and the product is water.
- Reactants: Hydrogen (H₂) and Oxygen (O₂)
- Product: Water (H₂O)
সম্পর্কিত প্রশ্ন
জ্ঞান পরীক্ষা করুন
Identify the reactant 'R'
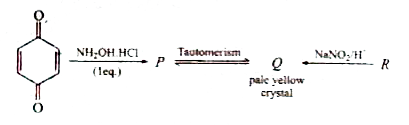 A
A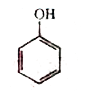 B
B C
C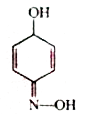 D
D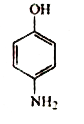
A reaction in which reactants (R ) are converted into products (P) follows second order kinetics. If concentration of R is increased by four times, what will be the increase in the rate formation of p?
A9 timesB4 timesC16 timesD8 times
Differentiate between :
Reactants and products
View SolutionAt chemical equilibrium , the concentration of all reactants and products are equal
View SolutionWhich of the following is true for a reaction in which all the reactant & product are liquids
View SolutionA chemical equation tells the actual number of moles of reactants and products.
View SolutionHow does fuel cell operate
View SolutionIn a reaction, all reactant and products are liquid, then
View Solution

