Which out of NH3 and NF3 has higher dipole moment and why?
To determine which molecule, NH3 (ammonia) or NF3 (nitrogen trifluoride), has a higher dipole moment, we need to analyze the molecular structure and the electronegativity of the atoms involved.
1. Understanding Dipole Moment:
The dipole moment is a measure of the separation of positive and negative charges in a molecule. It depends on the electronegativity of the atoms and the geometry of the molecule.
2. Molecular Geometry:
- For NH3(Ammonia):
- The nitrogen atom is at the center with three hydrogen atoms bonded to it and one lone pair of electrons.
- The molecular shape is trigonal pyramidal due to the lone pair.
- For NF3(NitrogenTrifluoride):
- Similar to ammonia, nitrogen is at the center with three fluorine atoms bonded to it and one lone pair of electrons.
- The shape is also trigonal pyramidal.
3. Electronegativity:
- The electronegativity values (Pauling scale) are approximately:
- Nitrogen (N) = 3.0
- Hydrogen (H) = 2.1
- Fluorine (F) = 4.0
- In NH3, the dipole moments from the N-H bonds point towards nitrogen, as nitrogen is more electronegative than hydrogen.
- In NF3, the dipole moments from the N-F bonds point towards fluorine, as fluorine is more electronegative than nitrogen.
4. Direction of Dipole Moments:
- In NH3:
- The dipole moments from the N-H bonds add up in the direction of the lone pair (upward), resulting in a net dipole moment pointing upwards.
- In NF3:
- The dipole moments from the N-F bonds point towards fluorine (downward), and since all three dipole moments are in the same direction, they add up.
5. Comparing the Dipole Moments:
- In NH3, the dipole moments from the N-H bonds oppose the lone pair's contribution, leading to a smaller net dipole moment.
- In NF3, the dipole moments from the N-F bonds reinforce each other, leading to a larger net dipole moment.
6. Conclusion:
- Therefore, NF3 has a higher dipole moment than NH3 because the dipole moments in NF3 add constructively, while in NH3, they partially cancel out.
Final Answer:
NF3 has a higher dipole moment than NH3 because the dipole moments from the N-F bonds reinforce each other, while in NH3, the dipole moments from the N-H bonds oppose the effect of the lone pair.
The dipole moment is a measure of the separation of positive and negative charges in a molecule. It depends on the electronegativity of the atoms and the geometry of the molecule.
- For NH3(Ammonia):
- The nitrogen atom is at the center with three hydrogen atoms bonded to it and one lone pair of electrons.
- The molecular shape is trigonal pyramidal due to the lone pair.
- For NF3(NitrogenTrifluoride):
- Similar to ammonia, nitrogen is at the center with three fluorine atoms bonded to it and one lone pair of electrons.
- The shape is also trigonal pyramidal.
- The electronegativity values (Pauling scale) are approximately:
- Nitrogen (N) = 3.0
- Hydrogen (H) = 2.1
- Fluorine (F) = 4.0
- In NH3, the dipole moments from the N-H bonds point towards nitrogen, as nitrogen is more electronegative than hydrogen.
- In NF3, the dipole moments from the N-F bonds point towards fluorine, as fluorine is more electronegative than nitrogen.
- In NH3:
- The dipole moments from the N-H bonds add up in the direction of the lone pair (upward), resulting in a net dipole moment pointing upwards.
- In NF3:
- The dipole moments from the N-F bonds point towards fluorine (downward), and since all three dipole moments are in the same direction, they add up.
- In NH3, the dipole moments from the N-H bonds oppose the lone pair's contribution, leading to a smaller net dipole moment.
- In NF3, the dipole moments from the N-F bonds reinforce each other, leading to a larger net dipole moment.
- Therefore, NF3 has a higher dipole moment than NH3 because the dipole moments in NF3 add constructively, while in NH3, they partially cancel out.
NF3 has a higher dipole moment than NH3 because the dipole moments from the N-F bonds reinforce each other, while in NH3, the dipole moments from the N-H bonds oppose the effect of the lone pair.
Topper's Solved these Questions
Similar Questions
Knowledge Check
Which has maximum dipole moment ?
A B
B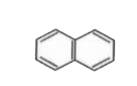 C
C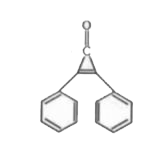 D
D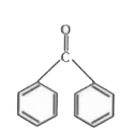
Out of CS2 and OCS which have higher dipole moment and why?
View SolutionAmong NH3andPH3 which is expected to have a higher dipole moment and why?
View SolutionWhich one of the following has the highest dipole moment ?
View SolutionWhich has maximum dipole moment ?
View SolutionWhich of the following has the highest dipole moment ?
View SolutionWhich has maximum dipole moment?
View SolutionOut of KCI and CaO, which has higher lattice energy and why?
View Solution
