The order of increasing ionic radius of the following is:
K+<Li+<Mg2+<Al3+
K+<Mg2+<Li+<Al3+
Li+<K+Mg2+<Al3+
Al3+<Mg2+<Li+<K+
The correct Answer is:D
Step by step video solution for The order of increasing ionic radius of the following is: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- PERIODIC PROPERTIESBOOK - VK JAISWAL ENGLISHCHAPTER - PERIODIC PROPERTIESEXERCISE - Level 3 (Passage Type)
81 Videos
- PERIODIC PROPERTIESBOOK - VK JAISWAL ENGLISHCHAPTER - PERIODIC PROPERTIESEXERCISE - ONE OR MORE ANSWERS IN/ARE CORRECT
94 Videos
- PERIODIC PROPERTIESBOOK - VK JAISWAL ENGLISHCHAPTER - PERIODIC PROPERTIESEXERCISE - MATCH THE COLUMN
11 Videos
- p-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - p-BLOCK ELEMENTSEXERCISE - SUBJECTIVE PROBLEMS
35 Videos
- QUALITATIVE INORGANIC ANALYSISBOOK - VK JAISWAL ENGLISHCHAPTER - QUALITATIVE INORGANIC ANALYSISEXERCISE - SUBJECTIVE PROBLEMS
3 Videos
Similar Questions
Knowledge Check
Increasing order of the following stability is :
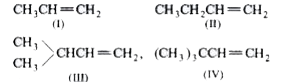 AI>II>III>IVBI>III>II>IVCI>IV>III>IIDIV>III>II>I
AI>II>III>IVBI>III>II>IVCI>IV>III>IIDIV>III>II>I
The correct order of decreasing ionic radii among the following isoelectronic species is
View SolutionFor Na+,Mg2+,F−andO2−, the correct order of increasing ionic radii is :
View SolutionThe correct order of ionic radii of the following species is
View SolutionCorrect order of ionic radii among the following is
View SolutionWhat is the correct increasing order of ionic atomic radii in the following ?
View SolutionWhen the following five anions are arranged in order of decreasing ionic radius , the correct sequence is :
View SolutionIdentify the correct order in which the covalent radius of the following elements increases ?
(i) Ti (ii) Ca
(iii) Sc
View Solution

