Ionization energies of five elements in kcal/mol are given below:
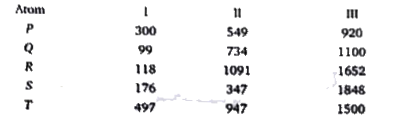
Q. If Q reacts with fluorine and oxyge, the molecular formula of fluoride and oxide will be respectively:
QF3,Q2Q3
QF,Q2O
QF2,QO
none of these
The correct Answer is:B
Q is alkali metal as it show group in I.E2 value.
Topper's Solved these Questions
- PERIODIC PROPERTIESBook:VK JAISWAL ENGLISHChapter:PERIODIC PROPERTIESExercise:ONE OR MORE ANSWERS IN/ARE CORRECT
Explore 94 Videos
- PERIODIC PROPERTIESBook:VK JAISWAL ENGLISHChapter:PERIODIC PROPERTIESExercise:MATCHTHE COLUMN
Explore 11 Videos
- PERIODIC PROPERTIESBook:VK JAISWAL ENGLISHChapter:PERIODIC PROPERTIESExercise:Level 2
Explore 101 Videos
- p-BLOCK ELEMENTSBook:VK JAISWAL ENGLISHChapter:p-BLOCK ELEMENTSExercise:SUBJECTIVE PROBLEMS
Explore 35 Videos
- QUALITATIVE INORGANIC ANALYSISBook:VK JAISWAL ENGLISHChapter:QUALITATIVE INORGANIC ANALYSISExercise:SUBJECTIVE PROBLEMS
Explore 3 Videos
Similar Questions
Ionization energies of five elements in kcal/mol are given below:
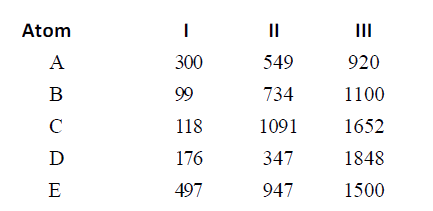 .
.
If B react with fluorine and oxygen, the molecular formula of fluoride and oxide will be respectively :
View SolutionIonization energies of five elements in kcal/mol are given below:
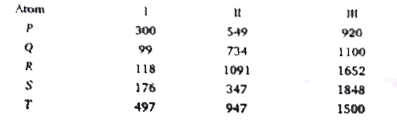
Q. Which element is a noble gas?
View SolutionIonization energies of five elements in kcal/mol are given below:
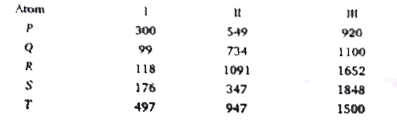
Q. Which element is a noble gas?
View SolutionIonization energies of five elements in kcal/mol are given below:
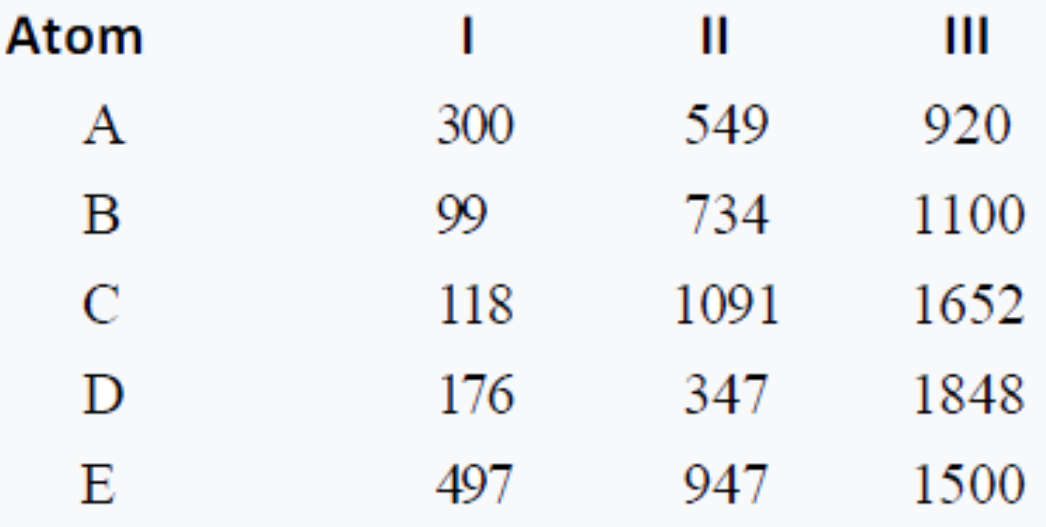 .
.
Which element is a noble gas ?
View SolutionIonization energies of five elements in kcal/mol are given below :
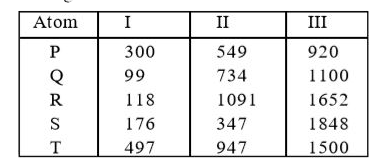
Which element is a noble gas ?
View SolutionIonization energies of five elements in kcal/mol are given below:
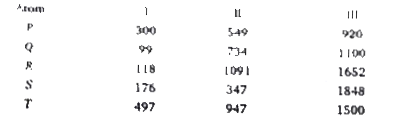
Q. Which element form stable unipositve ion?
View SolutionIonization energies of five elements in kcal/mol are given below:
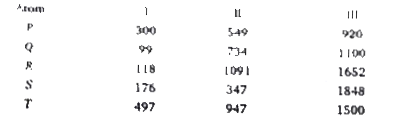
Q. Which element form stable unipositve ion?
View SolutionIonization energies of five elements in kcal/mol are given below:
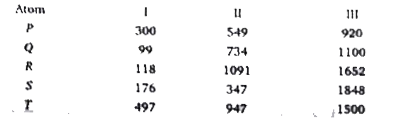
Q. Which is a non-metal (excluding noble gas)?
View SolutionIonization energies of five elements in kcal/mol are given below:
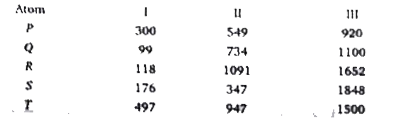
Q. Which is a non-metal (excluding noble gas)?
View SolutionIonization energies of five elements in kcal/mol are given below :
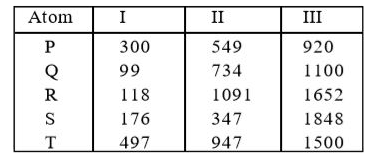
The element form stable unipositive ion?
View Solution
