Consider following four compounds:
(i) CxOy
(ii) CxOy+1
(iii) Cx+2Oy+1 and (iv) Cx+11Oy+8,
if "x=y=1", then calculate the vlaue of |p-q|, where p and q are total number of sp2 and hybridized carbon atoms respectively in given four compounds.
The correct Answer is:7
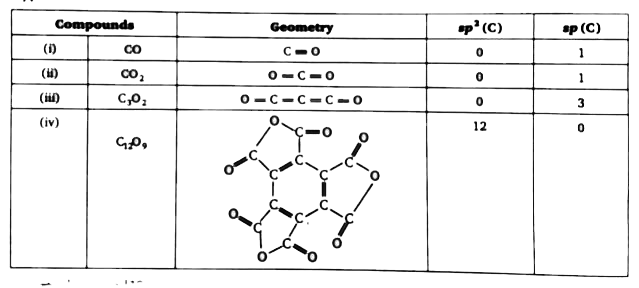
⇒|p−q|=|12−5|=7
Topper's Solved these Questions
Similar Questions
Assertion : C3O2 is non-polar molecule.
Reason : Terminal pi-bonds of the molecule are lying in different planes.
View SolutionConsider following compounds A to E :
(A) XeFn (B)XeF+(n+1) (C)XeF−(n+1) (D)XeF(n+2)
(E ) XeF2−(n+4),
If value of n is 4, then calculate value of p÷q here, 'p' is total number of bond pair and 'q' is total number of lone pair on central atoms of compounds (A) to (E ).
View SolutionThe solution of ((dy)/(dx))^(2)+(2x+y)(dy)/(dx)+2xy=0, is (i) (y+x^(2)-c_(1))(x+log y+y^(2)+c_(2))( (ii) (y+x^(2)-c_(1))(x-log y-c_(2))( iii) (y+x^(2)-c_(1))(x+log y-c_(2))( iv) (y+x^(2)-c_(1))(3x+log y-c_(2))
View SolutionConsider following three compounds:
(i) CxOy (ii) CxOy+1 (iii) Cx+2Oy+1
If x=y=1, then calcuate the value of |p−q|, where p and q are total number of sp2 and sp hybridised carbon atoms respectively in given three compounds.
View SolutionThe ratio of mass percent of C and H of an organic compound (CXHYOZ) is 6 : 1 If one molecule of the above compound (CXHYOZ)contains half as much oxygen as required to burn one molecule of compound CXHY completely to CO2 and H2O. The empirical formula of compound CXHYOZ is
View SolutionThe ration of mass per cent of C and H of an organic compound (C_(x)H_(y)O_(z)) "is"6:1 . If one molecule of the above compound (C_(x)H_(Y)O_(z)) contains half as much oxygen as required to burn one molecule of compound C_(x)H_(Y) compleltely to CO_(2) and H_(2)O . The empirial formula of compound C_(x)H_(y)O_(z) is:
View SolutionEvaluate ∣∣ ∣∣.xC1.xC2.xC3.yC1.yC2.yC3.xC1.zC2.zC3∣∣ ∣∣
View SolutionThe ration of mass per cent of C and H of an organic compound (CxHyOz)is6:1. If one molecule of the above compound (CxHYOz) contains half as much oxygen as required to burn one molecule of compound CxHY compleltely to CO2andH2O. The empirial formula of compound CxHyOz is:
View SolutionConsider following four compounds: (i) C_(x) O_(y) (ii) C_(x)O_(y+1) (iii) C_(x+2)O_(y+1) and (iv) C_(x+11)O_(y+8) , if "x=y=1", then calculate the vlaue of |p-q|, where p and q are total number of sp^(2) and hybridized carbon atoms respectively in given four compounds.
View SolutionC_(6)H_(6) +O_(3)to Xoverset(Zn//H_(2)O)to 1 Y , X and Y are respectively
View SolutionTotal number of covalent bonds in C_(3)O_(2) is x and y is the number of sp - hybridised atom. Find the sum of x+y ?
View SolutionConsider following four compounds:
(i) CxOy
(ii) CxOy+1
(iii) Cx+2Oy+1 and (iv) Cx+11Oy+8,
if "x=y=1", then calculate the vlaue of |p-q|, where p and q are total number of sp2 and hybridized carbon atoms respectively in given four compounds.
View SolutionEvaluate ∣∣ ∣∣.xC1.xC2.xC3.yC1.yC2.yC3.xC1.zC2.zC3∣∣ ∣∣
View SolutionThe ratio of mass percent of C and H of an organic compound (CXHYOZ) is 6: 1. If one molecule of the above compound (CXHYOZ) contains half as much oxygen as required to burn one molecule of compound CXHY completely to CO2andH2O. The empirical formula of compound CXHYOZ is.
View SolutionThe ratio of mass percent of C and H of an organic compound (CXHYOZ) is 6: 1. If one molecule of the above compound (CXHYOZ) contains half as much oxygen as required to burn one molecule of compound CXHY completely to CO2andH2O. The empirical formula of compound CXHYOZ is.
View Solutionकिसी कार्बनिक यौगिक (CxHyOz) में C तथा H का द्रव्यमान प्रतिशत 6:1 अनुपात है। यदि यौगिक (CxHy) के एक अणु के पूर्ण दहन से CO2 तथा H2O प्राप्त करने के लिये यौगिक (CxHyOz) के एक अणु में स्थित ऑक्सीजन की मात्रा अणु की आधी प्रयुक्त होती है, तो यौगिक CxHyOz का मूलानुपाती सूत्र होगा
View Solution
