Which one of the following has lowest value of paramagnetic behaviour?
[Cr(CN)6]3−
[Mn(CN)6]3−
[Fe(CN)6]3−
[Co(CN)6]3−
The correct Answer is:D
To determine which of the given coordination compounds has the lowest value of paramagnetic behavior, we will follow these steps:
Step 1: Identify the Coordination Compounds
We need to identify the coordination compounds provided in the question. Let's assume we have the following complexes:
1. (Cr(CN)6)³⁻
2. (Mn(CN)6)³⁻
3. (Co(CN)6)³⁻
4. (Fe(CN)6)³⁻
Step 2: Determine the Oxidation States
Next, we will calculate the oxidation states of the central metal ions in each complex:
- For (Cr(CN)6)³⁻: CN is a -1 ligand, so the oxidation state of Cr is +3.
- For (Mn(CN)6)³⁻: CN is a -1 ligand, so the oxidation state of Mn is +3.
- For (Co(CN)6)³⁻: CN is a -1 ligand, so the oxidation state of Co is +3.
- For (Fe(CN)6)³⁻: CN is a -1 ligand, so the oxidation state of Fe is +3.
Step 3: Write the Electronic Configurations
Now we will write the electronic configurations for the metals in their +3 oxidation states:
- Chromium (Cr): Atomic number 24 → (Ar) 4s² 3d⁴ → In +3 state: (Ar) 3d³ (3 unpaired electrons)
- Manganese (Mn): Atomic number 25 → (Ar) 4s² 3d⁵ → In +3 state: (Ar) 3d⁴ (4 unpaired electrons)
- Cobalt (Co): Atomic number 27 → (Ar) 4s² 3d⁷ → In +3 state: (Ar) 3d⁶ (4 unpaired electrons)
- Iron (Fe): Atomic number 26 → (Ar) 4s² 3d⁶ → In +3 state: (Ar) 3d⁵ (5 unpaired electrons)
Step 4: Determine the Number of Unpaired Electrons
Now we will determine the number of unpaired electrons in each case:
- (Cr(CN)6)³⁻: 3 unpaired electrons
- (Mn(CN)6)³⁻: 4 unpaired electrons
- (Co(CN)6)³⁻: 4 unpaired electrons
- (Fe(CN)6)³⁻: 5 unpaired electrons
Step 5: Analyze the Ligand Field Strength
Since CN⁻ is a strong field ligand, it causes pairing of electrons. Thus, we need to consider that:
- In the case of (Cr(CN)6)³⁻, the 3 unpaired electrons can pair up due to the strong field nature of CN⁻.
- The same applies to the other complexes, but the extent of pairing will depend on the number of unpaired electrons.
Step 6: Conclusion
After analyzing the number of unpaired electrons:
- (Cr(CN)6)³⁻: can become diamagnetic (0 unpaired electrons)
- (Mn(CN)6)³⁻: 4 unpaired electrons
- (Co(CN)6)³⁻: 4 unpaired electrons
- (Fe(CN)6)³⁻: 5 unpaired electrons
The complex with the lowest value of paramagnetic behavior is (Cr(CN)6)³⁻, which can become diamagnetic due to the strong field ligand CN⁻.
Final Answer
The coordination compound with the lowest value of paramagnetic behavior is (Cr(CN)6)³⁻.
We need to identify the coordination compounds provided in the question. Let's assume we have the following complexes:
1. (Cr(CN)6)³⁻
2. (Mn(CN)6)³⁻
3. (Co(CN)6)³⁻
4. (Fe(CN)6)³⁻
Next, we will calculate the oxidation states of the central metal ions in each complex:
- For (Cr(CN)6)³⁻: CN is a -1 ligand, so the oxidation state of Cr is +3.
- For (Mn(CN)6)³⁻: CN is a -1 ligand, so the oxidation state of Mn is +3.
- For (Co(CN)6)³⁻: CN is a -1 ligand, so the oxidation state of Co is +3.
- For (Fe(CN)6)³⁻: CN is a -1 ligand, so the oxidation state of Fe is +3.
Now we will write the electronic configurations for the metals in their +3 oxidation states:
- Chromium (Cr): Atomic number 24 → (Ar) 4s² 3d⁴ → In +3 state: (Ar) 3d³ (3 unpaired electrons)
- Manganese (Mn): Atomic number 25 → (Ar) 4s² 3d⁵ → In +3 state: (Ar) 3d⁴ (4 unpaired electrons)
- Cobalt (Co): Atomic number 27 → (Ar) 4s² 3d⁷ → In +3 state: (Ar) 3d⁶ (4 unpaired electrons)
- Iron (Fe): Atomic number 26 → (Ar) 4s² 3d⁶ → In +3 state: (Ar) 3d⁵ (5 unpaired electrons)
Now we will determine the number of unpaired electrons in each case:
- (Cr(CN)6)³⁻: 3 unpaired electrons
- (Mn(CN)6)³⁻: 4 unpaired electrons
- (Co(CN)6)³⁻: 4 unpaired electrons
- (Fe(CN)6)³⁻: 5 unpaired electrons
Since CN⁻ is a strong field ligand, it causes pairing of electrons. Thus, we need to consider that:
- In the case of (Cr(CN)6)³⁻, the 3 unpaired electrons can pair up due to the strong field nature of CN⁻.
- The same applies to the other complexes, but the extent of pairing will depend on the number of unpaired electrons.
After analyzing the number of unpaired electrons:
- (Cr(CN)6)³⁻: can become diamagnetic (0 unpaired electrons)
- (Mn(CN)6)³⁻: 4 unpaired electrons
- (Co(CN)6)³⁻: 4 unpaired electrons
- (Fe(CN)6)³⁻: 5 unpaired electrons
The coordination compound with the lowest value of paramagnetic behavior is (Cr(CN)6)³⁻.
টপার এই প্রশ্নগুলো সমাধান করেছে
- CO-ORDINATION COMPOUNDSBook:VK JAISWAL ENGLISHChapter:CO-ORDINATION COMPOUNDSExercise:LEVEL 2
Explore 143 Videos
- CO-ORDINATION COMPOUNDSBook:VK JAISWAL ENGLISHChapter:CO-ORDINATION COMPOUNDSExercise:LEVEL 3 (PASSAGE TYPE)
Explore 96 Videos
- CHEMICAL BONDING (BASIC)Book:VK JAISWAL ENGLISHChapter:CHEMICAL BONDING (BASIC)Exercise:SUBJECTIVE PROBLEMS
Explore 49 Videos
- d-BLOCK ELEMENTSBook:VK JAISWAL ENGLISHChapter:d-BLOCK ELEMENTSExercise:SUBJECTIVE PROBLEMS
Explore 6 Videos
সম্পর্কিত প্রশ্ন
জ্ঞান পরীক্ষা করুন
Which of the following has lowest pKa value ?
A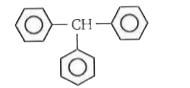 B
B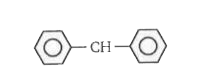 C
C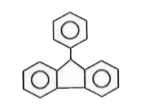 D
D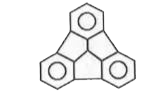
Which one of the following has lowest heat of combustion?
A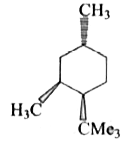 B
B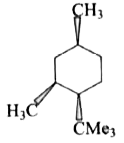 C
C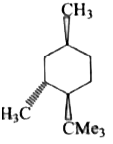 D
D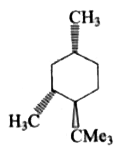
Which of the following acids has lowest pKa value?
ACH2∣NO2−O∣∣C−OHBCH2∣NO2−CH2−O∣∣C−OHCCH2∣Cl−O∣∣C−OHDCH3−Cl∣C∣Cl−O∣∣C−OH
The d-electron configurations of Cr2+,Mn2+,Fe2+ and Co2+ are d4,d5,d6 and d7 respectively. Which one of the following will exhibit minimum paramagnetic behaviour?
View SolutionWhich of the following has lowest percentage of carbon?
View SolutionWhich of the following has the lowest density?
View SolutionWhich of the following has largest paramagnetism ?
View SolutionThe lowest value of paramagnetism is shown by
View Solution
