For a sponaneous reaction, the free energy change must be negative, ΔG=ΔH−TΔS,ΔH is the enthalpy change during the reaction. T is the absolute temperature, and ΔS is the change in entropy during the reaction. Consider a reaction such as the formation of an oxide
M+O2→MO
Dioxygen is used up in the course of this reaction. Gases have a more random structure (less ordered) than liquid or solids. Consequently gases have a higher entropy than liquids and solids. In this reaction S (entropy or randomness) decreases, hence ΔS is negative. Thus, if the temperature is raised then TΔS becomes more negative,Since, TΔS is substracted in the equation, then ΔG becomes less negative. Thus, the free energy change increases with the increase in temperature.
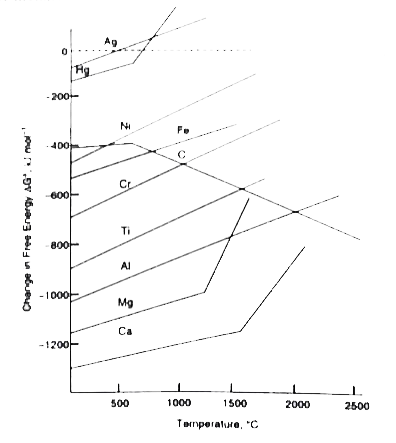
The free energy changes that occur when one mole of common reactant (in this case dioxygen) is used may be plotted graphically aginst temperature for a number of reactions of metals to their oxides. The following plot is called an Ellingham diagram for metal oxide. Understanding of Ellingham diagram is extremely important for the efficient extraction of metals.
For the conversion of Ca(s) to Ca(s) which of the following represent the ΔG vs. T ?
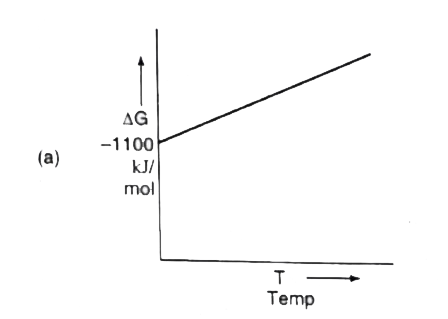
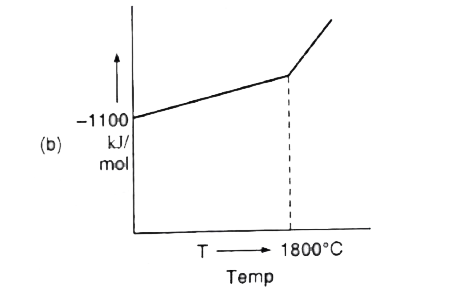
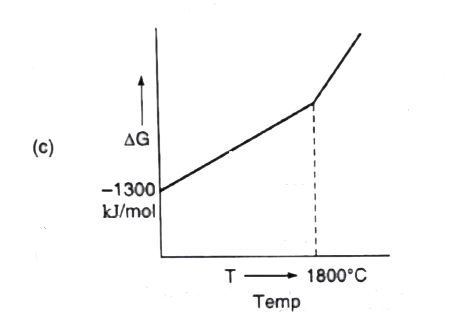
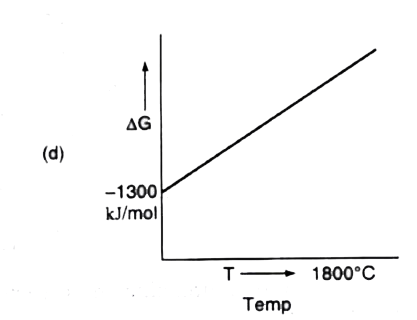
The correct Answer is:C
Step by step video solution for For a sponaneous reaction, the free energy change must be negative, Delta G=Delta H-T Delta S, Delta H is the enthalpy change during the reaction. T is the absolute temperature, and Delta S is the change in entropy during the reaction. Consider a reaction such as the formation of an oxide M+O_(2) to MO Dioxygen is used up in the course of this reaction. Gases have a more random structure (less ordered) than liquid or solids. Consequently gases have a higher entropy than liquids and solids. In this reaction S (entropy or randomness) decreases, hence Delta S is negative. Thus, if the temperature is raised then T Delta S becomes more negative,Since, TDelta S is substracted in the equation, then Delta G becomes less negative. Thus, the free energy change increases with the increase in temperature. The free energy changes that occur when one mole of common reactant (in this case dioxygen) is used may be plotted graphically aginst temperature for a number of reactions of metals to their oxides. The following plot is called an Ellingham diagram for metal oxide. Understanding of Ellingham diagram is extremely important for the efficient extraction of metals. For the conversion of Ca(s) to Ca(s) which of the following represent the Delta G vs. T ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- METALLURGYBOOK - VK JAISWAL ENGLISHCHAPTER - METALLURGYEXERCISE - Level 3 (Passive 2)
6 Videos
- METALLURGYBOOK - VK JAISWAL ENGLISHCHAPTER - METALLURGYEXERCISE - Level 3 (Passive 3)
6 Videos
- METALLURGYBOOK - VK JAISWAL ENGLISHCHAPTER - METALLURGYEXERCISE - Level 2
86 Videos
- ENVIRONMENTAL CHEMISTRYBOOK - VK JAISWAL ENGLISHCHAPTER - ENVIRONMENTAL CHEMISTRYEXERCISE - ASSERTION-REASON TYPE QUESTIONS
14 Videos
- p-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - p-BLOCK ELEMENTSEXERCISE - SUBJECTIVE PROBLEMS
35 Videos
Similar Questions
For a sponaneous reaction, the free energy change must be negative, ΔG=ΔH−TΔS,ΔH is the enthalpy change during the reaction. T is the absolute temperature, and ΔS is the change in entropy during the reaction. Consider a reaction such as the formation of an oxide
M+O2→MO
Dioxygen is used up in the course of this reaction. Gases have a more random structure (less ordered) than liquid or solids. Consequently gases have a higher entropy than liquids and solids. In this reaction S (entropy or randomness) decreases, hence ΔS is negative. Thus, if the temperature is raised then TΔS becomes more negative,Since, TΔS is substracted in the equation, then ΔG becomes less negative. Thus, the free energy change increases with the increase in temperature.
The free energy changes that occur when one mole of common reactant (in this case dioxygen) is used may be plotted graphically aginst temperature for a number of reactions of metals to their oxides. The following plot is called an Ellingham diagram for metal oxide. Understanding of Ellingham diagram is extremely important for the efficient extraction of metals.
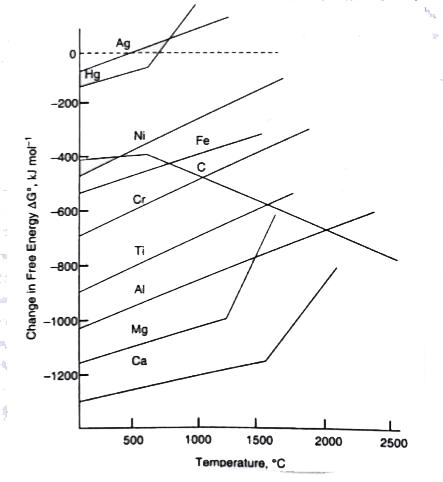
As per the Ellingham diagram of oxides which of the following conclusion is true ?
View SolutionFor a sponaneous reaction, the free energy change must be negative, ΔG=ΔH−TΔS,ΔH is the enthalpy change during the reaction. T is the absolute temperature, and ΔS is the change in entropy during the reaction. Consider a reaction such as the formation of an oxide
M+O2→MO
Dioxygen is used up in the course of this reaction. Gases have a more random structure (less ordered) than liquid or solids. Consequently gases have a higher entropy than liquids and solids. In this reaction S (entropy or randomness) decreases, hence ΔS is negative. Thus, if the temperature is raised then TΔS becomes more negative,Since, TΔS is substracted in the equation, then ΔG becomes less negative. Thus, the free energy change increases with the increase in temperature.
The free energy changes that occur when one mole of common reactant (in this case dioxygen) is used may be plotted graphically aginst temperature for a number of reactions of metals to their oxides. The following plot is called an Ellingham diagram for metal oxide. Understanding of Ellingham diagram is extremely important for the efficient extraction of metals.
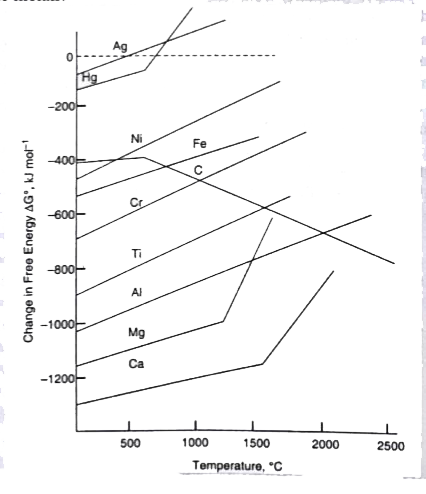
Free energy change of Hg and Mg for the convertion to oxides the slpe of ΔG vsT has been changed above the boiling points of the given metal because :
View SolutionFor a sponaneous reaction, the free energy change must be negative, ΔG=ΔH−TΔS,ΔH is the enthalpy change during the reaction. T is the absolute temperature, and ΔS is the change in entropy during the reaction. Consider a reaction such as the formation of an oxide
M+O2→MO
Dioxygen is used up in the course of this reaction. Gases have a more random structure (less ordered) than liquid or solids. Consequently gases have a higher entropy than liquids and solids. In this reaction S (entropy or randomness) decreases, hence ΔS is negative. Thus, if the temperature is raised then TΔS becomes more negative,Since, TΔS is substracted in the equation, then ΔG becomes less negative. Thus, the free energy change increases with the increase in temperature.
The free energy changes that occur when one mole of common reactant (in this case dioxygen) is used may be plotted graphically aginst temperature for a number of reactions of metals to their oxides. The following plot is called an Ellingham diagram for metal oxide. Understanding of Ellingham diagram is extremely important for the efficient extraction of metals.
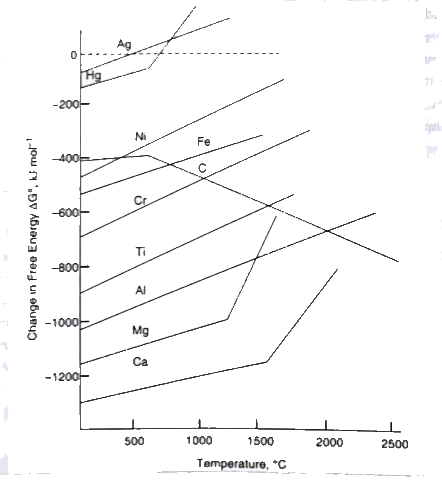
Which of the following elements can be prepared by heating the oxide above 400∘C ?
View SolutionThe free energy change, ΔG = 0, when
View SolutionWhich of the following reactions is ΔH less than ΔE ?
View SolutionIn which reaction(s), ΔS in negative?
View SolutionWhich of the following reactions represents ΔH (hydration) :-
View SolutionFor an exothermic reaction to be spontaneous (ΔS = negative)
View SolutionIn which of the following changes, ΔH is always negative :
View SolutionFor which reaction, (ΔH>ΔU)? ( ΔH= change in enthalpy,ΔU = change in internal energy)
View Solution

