Which of the following is / are correctly matched ?
Column-I(Metals)Calumn-II(Process used for extraction)TitaniumKroll process
Column-I(Metals)Calumn-II(Process used for extraction)AluminiumBaeyer process
Column-I(Metals)Calumn-II(Process used for extraction)ChromiumThermite process
Column-I(Metals)Calumn-II(Process used for extraction)SilverMac-Arthur cyanide process
The correct Answer is:A, C, D
To determine which of the given metal-extraction process pairs are correctly matched, we will analyze each pair step by step.
1. Titanium - Kroll Process:
- The Kroll process is indeed used for the extraction of titanium. It involves the reduction of titanium tetrachloride (TiCl4) using magnesium at high temperatures (around 800°C).
- Reaction:
TiCl4+2Mg→Ti+2MgCl2
- Conclusion: This pair is correctly matched.
2. Aluminium - Bayer Process:
- The Bayer process is used for refining bauxite to produce alumina (Al2O3), which is then used to extract aluminum via electrolysis.
- However, the extraction of aluminum itself is done through the Hall-Héroult process, not the Bayer process.
- Conclusion: This pair is incorrectly matched.
3. Chromium - Thermite Process:
- The thermite process involves the reduction of metal oxides using aluminum as a reducing agent. It is indeed used for extracting chromium from its oxide.
- Reaction:
Cr2O3+2Al→2Cr+Al2O3
- Conclusion: This pair is correctly matched.
4. Silver - MacArthur-Forrest (Cyanide) Process:
- The MacArthur-Forrest process, also known as the cyanide process, is used for extracting silver (and gold) from ores by dissolving them in a dilute solution of sodium cyanide or potassium cyanide.
- Conclusion: This pair is correctly matched.
Summary of Matches:
- Correctly Matched:
- 1. Titanium - Kroll Process
- 3. Chromium - Thermite Process
- 4. Silver - MacArthur-Forrest Process
- Incorrectly Matched:
- 2. Aluminium - Bayer Process
Final Answer:
The pairs that are correctly matched are:
- Titanium with Kroll Process
- Chromium with Thermite Process
- Silver with MacArthur-Forrest Process
- The Kroll process is indeed used for the extraction of titanium. It involves the reduction of titanium tetrachloride (TiCl4) using magnesium at high temperatures (around 800°C).
- Reaction:
TiCl4+2Mg→Ti+2MgCl2
- Conclusion: This pair is correctly matched.
- The Bayer process is used for refining bauxite to produce alumina (Al2O3), which is then used to extract aluminum via electrolysis.
- However, the extraction of aluminum itself is done through the Hall-Héroult process, not the Bayer process.
- Conclusion: This pair is incorrectly matched.
- The thermite process involves the reduction of metal oxides using aluminum as a reducing agent. It is indeed used for extracting chromium from its oxide.
- Reaction:
Cr2O3+2Al→2Cr+Al2O3
- Conclusion: This pair is correctly matched.
- The MacArthur-Forrest process, also known as the cyanide process, is used for extracting silver (and gold) from ores by dissolving them in a dilute solution of sodium cyanide or potassium cyanide.
- Conclusion: This pair is correctly matched.
- Correctly Matched:
- 1. Titanium - Kroll Process
- 3. Chromium - Thermite Process
- 4. Silver - MacArthur-Forrest Process
- 2. Aluminium - Bayer Process
The pairs that are correctly matched are:
- Titanium with Kroll Process
- Chromium with Thermite Process
- Silver with MacArthur-Forrest Process
Topper's Solved these Questions
- METALLURGYBOOK - VK JAISWAL ENGLISHCHAPTER - METALLURGYEXERCISE - MATCH THE COLUMN
20 Videos
- METALLURGYBOOK - VK JAISWAL ENGLISHCHAPTER - METALLURGYEXERCISE - ASSERTION-REASON TYPE QUESTIONS
29 Videos
- METALLURGYBOOK - VK JAISWAL ENGLISHCHAPTER - METALLURGYEXERCISE - Level 3 (Passive 7)
8 Videos
- ENVIRONMENTAL CHEMISTRYBOOK - VK JAISWAL ENGLISHCHAPTER - ENVIRONMENTAL CHEMISTRYEXERCISE - ASSERTION-REASON TYPE QUESTIONS
14 Videos
- p-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - p-BLOCK ELEMENTSEXERCISE - SUBJECTIVE PROBLEMS
35 Videos
Similar Questions
Column-I and Column-II contains four entries each. Entries of column-I are to be matched with some entries of column-II. Each entry of column-I may have the matching with one or more than one entries of column-II.
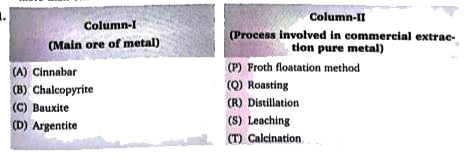
View SolutionAssertion : PbSiF6+H2SiF6+ gelatine is taken as electrolyte in electrolytic refining of lead.
Reason : Discharge potential of Pb2+ is less than H+.
View SolutionElectrolysis is an important technique for extraction of metals, and each ion of the solution needs a minimum voltage to get discharged and this value is expressed in terms of discharge potential. For some metal ions the discharge potentials follow the order given below :
Li+>K+>Ca2+>Na+>Mg2+>Al3+>Zn2+>Fe2+>Ni2+>H3O+>Cu2+>Hg2+2>Ag+>Au3+
For some anions the discharge potentials are in the order :
SO2−4>NO−3>OH−>Br−>I−
When aqueous solution of cupric bromide is electrolyzed the product obtained at cathode will be :
View SolutionAssertion (A) Green house effect was observed in houses used to grow plants and these are made of green glass.
Reason (R) Green house name has been given because glass houses are made of green glass.
View SolutionIn phosphorus acid, If X is number of non-bonding electron pairs. Y is number of σ-bonds and Z is number of π-bonds. Then calculate value of "YxZ-X".
View SolutionWhich of the following is not correctly matched ?
View SolutionWhich of the following is not correctly matched
View SolutionWhich of the following is correctly matched
View SolutionWhich of the following is correctly is correctly matched?
View SolutionWhich of following is not correctly matched?
View SolutionWhich of the following is / are correctly matched ?
View SolutionWhich of the following NOT CORRECTLY matched ?
View SolutionWhich of the following is/are correctly matched ?
View Solutionनिम्न में से कौनसा सही सुमेलित नहीं हैं
View Solutionनिम्न में से कौन गलत सुमेलित है
View Solutionनिम्न में से कौन सही सुमेलित नहीं है?
View Solution

