Which of the following cation does not give precipitate with H2S in neutral medium?
Fe3+
Cu2+
Bi3+
Ag+
The correct Answer is:A
Fe3+(aq.)+H2SRedox−−−→S⏐⏐↓+Fe2+(aq.)Neutral H2S−−−−−−−→ No ppt.
Topper's Solved these Questions
- TYPES OF REACTIONSBook:VK JAISWAL ENGLISHChapter:TYPES OF REACTIONSExercise:LEVEL 3
Explore 36 Videos
- TYPES OF REACTIONSBook:VK JAISWAL ENGLISHChapter:TYPES OF REACTIONSExercise:ONE OR MORE ANSWERS IS/ARE CORRECT
Explore 40 Videos
- TYPES OF REACTIONSBook:VK JAISWAL ENGLISHChapter:TYPES OF REACTIONSExercise:MATCH THE COLUMN
Explore 3 Videos
- s-BLOCK ELEMENTSBook:VK JAISWAL ENGLISHChapter:s-BLOCK ELEMENTSExercise:SUBJECTIVE PROBLEMS
Explore 2 Videos
Similar Questions
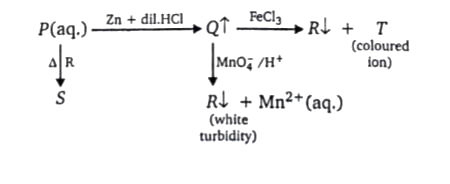
Q. Species P and S are respectively:
View SolutionWhich of the following combination of species can evolve O2?
View SolutionMatch the following columns

View Solution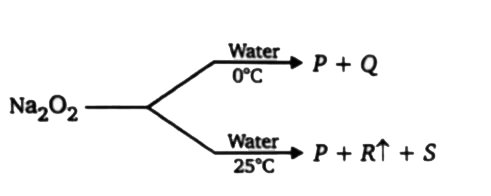
Calculate sum of bond order between same bonded atoms in Q and R compounds.
View SolutionAn aqueous solutio of a salt (A) gives white precipitate with NaCl solution and filtrate of this with H2S gives black precipitate . Then which one of the following cations should be present in
View SolutionWhich of the following cations give black precipitate with H2S gas as well as soluble with excess yellow sodium disulphide?
View SolutionWhich of the following cations form(s) black precipitate(s) with H_(2)S (g) ?
View SolutionA solution containing a group-IV cation gives a precipitate on passing, H_(2)S . A solution of this precipitate in dil. HCl produces a white precipitate with NaOH solution and bluish-white prcipitate with basic potassium ferrocyanide. The cation is :
View SolutionPrecipitate of group IV cations takes place when H2S is
View Solutionगुणात्मक विश्लेषण के II समूह में H2S को .......... माध्यम में प्रवाहित करना आवश्यक है अन्यथा समूह .......... के धनायनों के सल्फाइड अवक्षेपित हो जाएँगे।
View SolutionWhich of the following cation does not give precipitate with H2S in neutral medium?
View SolutionH2S is passed through the solution in an acididc medium to precipitate the sulphides of group II cations, but in an alkaline medium to precipitate the sulphides of group IV cations because:
View SolutionWhich of the following cation does not give precipitate with H2S in neutral medium?
View SolutionWhich of the following cation gives white precipitate with H_2S in alkaline medium ?
View SolutionWhich of the following cations is/are giving precipitate with "H_(2)S" in acidic medium?
1) \(Cu^{2+}\)
2) \(Ni^{+2}\)
3)\(Pb^{2+}\)
(4) \(Ba^{+2}\)
View Solutionकौन - सा धनायन अमोनिया की उपस्थिति में H2S द्वारा अवक्षेपित नहीं होगा ?
View Solution
