Consider three P, Q, R, salts among them P and Q salts have different cations annd also have different coloured polyatomic anion due to charge transfer phenomenon while P and R salts have same cation but have different anions. Salts R decomposes into an acidic gas an a basic gas.
Q. If salt Q underrgoes redox reaction with H2S in acidic medium then which of the following speies can not be possible product?
MnO2−4(aq.)
S
MnO2
Both (a) and (c)
The correct Answer is:D
To solve the question, we need to analyze the redox reaction between salt Q and H₂S in an acidic medium. Here’s a step-by-step breakdown of the solution:
Step 1: Identify the nature of salt Q
- Salt Q contains a different cation and a colored polyatomic anion. Given the context of the problem, we can assume that the polyatomic anion is likely to be MnO₄⁻ (permanganate), which is known for its strong oxidizing properties.
Hint: Look for common colored polyatomic anions that can act as oxidizing agents.
Step 2: Determine the role of H₂S
- In the reaction, H₂S acts as a reducing agent. This means that it will donate electrons and reduce the oxidizing agent (in this case, MnO₄⁻).
Hint: Remember that reducing agents are substances that lose electrons during a chemical reaction.
Step 3: Predict the products of the reaction
- The reaction between MnO₄⁻ and H₂S in acidic medium typically produces manganese ions (Mn²⁺) and elemental sulfur (S). The half-reaction for the reduction of MnO₄⁻ can be represented as:
MnO−4+8H++5e−→Mn2++4H2O
- H₂S gets oxidized to sulfur (S):
H2S→S+2H++2e−
Hint: Consider the oxidation states of the elements involved to identify the products formed.
Step 4: Analyze the possible products
- The question asks which species cannot be a possible product of the reaction. The potential products from the reaction are:
- Mn²⁺ (from MnO₄⁻)
- S (from H₂S)
- MnO₂ (which has Mn in the +4 oxidation state)
- MnO₄²⁻ (which is a reduced form of MnO₄⁻)
Step 5: Determine which species cannot be produced
- From the reaction, we can conclude:
- Mn²⁺ and S are indeed produced.
- MnO₂ cannot be produced because it requires a different oxidation state for manganese that is not achieved in this reaction.
- MnO₄²⁻ is also not produced because the reaction does not yield this species under acidic conditions.
Final Conclusion: The species that cannot be possible products are MnO₂ and MnO₄²⁻.
Final Answer:
The correct answer is option 4: both A (MnO₄²⁻) and C (MnO₂) cannot be possible products.
---
- Salt Q contains a different cation and a colored polyatomic anion. Given the context of the problem, we can assume that the polyatomic anion is likely to be MnO₄⁻ (permanganate), which is known for its strong oxidizing properties.
- In the reaction, H₂S acts as a reducing agent. This means that it will donate electrons and reduce the oxidizing agent (in this case, MnO₄⁻).
- The reaction between MnO₄⁻ and H₂S in acidic medium typically produces manganese ions (Mn²⁺) and elemental sulfur (S). The half-reaction for the reduction of MnO₄⁻ can be represented as:
MnO−4+8H++5e−→Mn2++4H2O
- H₂S gets oxidized to sulfur (S):
H2S→S+2H++2e−
- The question asks which species cannot be a possible product of the reaction. The potential products from the reaction are:
- Mn²⁺ (from MnO₄⁻)
- S (from H₂S)
- MnO₂ (which has Mn in the +4 oxidation state)
- MnO₄²⁻ (which is a reduced form of MnO₄⁻)
- From the reaction, we can conclude:
- Mn²⁺ and S are indeed produced.
- MnO₂ cannot be produced because it requires a different oxidation state for manganese that is not achieved in this reaction.
- MnO₄²⁻ is also not produced because the reaction does not yield this species under acidic conditions.
The correct answer is option 4: both A (MnO₄²⁻) and C (MnO₂) cannot be possible products.
Topper's Solved these Questions
- TYPES OF REACTIONSBOOK - VK JAISWAL ENGLISHCHAPTER - TYPES OF REACTIONSEXERCISE - ONE OR MORE ANSWERS IS/ARE CORRECT
40 Videos
- TYPES OF REACTIONSBOOK - VK JAISWAL ENGLISHCHAPTER - TYPES OF REACTIONSEXERCISE - SUBJECTIVE PROBLEMS
41 Videos
- TYPES OF REACTIONSBOOK - VK JAISWAL ENGLISHCHAPTER - TYPES OF REACTIONSEXERCISE - LEVEL 2
194 Videos
- s-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - s-BLOCK ELEMENTSEXERCISE - SUBJECTIVE PROBLEMS
2 Videos
Similar Questions
Which of the following combination of species can evolve O2?
View SolutionFind total number of reagnets which cann produce I2 from KI solution.
Conc. H2SO4, Hg(NO3)2solution, CuSO4 solution, Conc.H3PO4,
K2Cr2O7/H+, Cl2 Water, Pb(CH3COO)2 solution, Ca(OCl)Cl/H+, NaNO2+dil.HCl
View SolutionWhich of the following metal nitrate produces gaseous product when reacts with KCN solution?
View Solution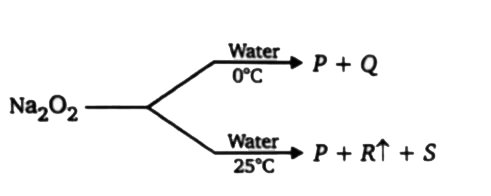
Calculate sum of bond order between same bonded atoms in Q and R compounds.
View SolutionIf salt Q undergoes redox reaction with H_(2)S in acidic medium then which of the following species can not be possible product?
View SolutionConsider three P, Q, R, salts among them P and Q salts have different cations annd also have different coloured polyatomic anion due to charge transfer phenomenon while P and R salts have same cation but have different anions. Salts R decomposes into an acidic gas an a basic gas.
Q. Salt R can not be:
View SolutionConsider three P, Q, R, salts among them P and Q salts have different cations annd also have different coloured polyatomic anion due to charge transfer phenomenon while P and R salts have same cation but have different anions. Salts R decomposes into an acidic gas an a basic gas. Q. Salt P decomposes on heating into a coloured solid, neutral gas a neutral vapour, then which of the following can not be the product of salt P after decomposition?
View SolutionConsider three P, Q, R, salts among them P and Q salts have different cations annd also have different coloured polyatomic anion due to charge transfer phenomenon while P and R salts have same cation but have different anions. Salts R decomposes into an acidic gas an a basic gas.
Q. If salt Q underrgoes redox reaction with H2S in acidic medium then which of the following speies can not be possible product?
View SolutionIn salts of polyatomic anion, as polarising power of cation increases, thermal stability of the salt decreases, and decomposed species may further undergo redox reaction. Q. Water soluble salt (x) was heated into three products A, B and C and B and C are two different paramagnetiic gases. A is red in hot condition, then salt (x) is:
View SolutionIn salts of polyatomic anion, as polarising power of cation increase, thermal stability of the salt decrease and decomposed species may further undergo redox reaction
Q. Water soluble salt(x) was heated into three products A, B and C and B and C are two different paramagnetic gases. A is red in hot condition, then salt(x) is :
View SolutionThe cation and anion of the electrolyte used in salt-bridge have ___________ mobility.
View SolutionIn salts of polyatomic anion, as polarising power of cation increase, thermal stability of the salt decrease and decomposed species may further undergo redox reaction Q. Water soluble salt(x) was heated into three products A, B and C and B and C are two different paramagnetic gases. A is red in hot condition, then salt(x) is :
View SolutionConsider three P, Q, R, salts among them P and Q salts have different cations annd also have different coloured polyatomic anion due to charge transfer phenomenon while P and R salts have same cation but have different anions. Salts R decomposes into an acidic gas an a basic gas.
Q. Salt R can not be:
View SolutionIf salt Q undergoes redox reaction with H2S in acidic medium then which of the following species can not be possible product?
View SolutionConsider three P, Q, R, salts among them P and Q salts have different cations annd also have different coloured polyatomic anion due to charge transfer phenomenon while P and R salts have same cation but have different anions. Salts R decomposes into an acidic gas an a basic gas.
Q. Salt P decomposes on heating into a coloured solid, neutral gas a neutral vapour, then which of the following can not be the product of salt P after decomposition?
View Solution

