Which of the following statements is wrong?
Ozone is produced in upper stratosphere by the action of UV rays on oxygen
Ozon can oxidize sulphur dioxide present in the atmosphere to sulphur trioxide
Ozone hole is thining of ozone layer present in stratosphere
none of these
The correct Answer is:D
To determine which statement is wrong, we will analyze each statement one by one.
Step 1: Analyze the First Statement
Statement: Ozone is produced in the upper stratosphere by the action of UV rays on oxygen.
Explanation: This statement is correct. Ozone is indeed produced in the upper stratosphere through a process where ultraviolet (UV) rays break down molecular oxygen (O2) into individual oxygen atoms (O). These oxygen atoms can then react with other O2 molecules to form ozone (O3).
Step 2: Analyze the Second Statement
Statement: Ozone can oxidize Sulphur dioxide present in the atmosphere to Sulphur trioxide.
Explanation: This statement is also correct. Ozone is a strong oxidizing agent and can react with sulfur dioxide (SO2) to form sulfur trioxide (SO3). This reaction can occur in the presence of UV radiation.
Step 3: Analyze the Third Statement
Statement: Ozone hole is thinning of the ozone layer present in the stratosphere.
Explanation: This statement is correct as well. The term "ozone hole" refers to the significant reduction in ozone concentration in the stratosphere, primarily caused by human-made chemicals such as chlorofluorocarbons (CFCs).
Step 4: Analyze the Fourth Statement
Statement: None of these.
Explanation: This statement implies that none of the previous statements are wrong. Since we have established that all previous statements are correct, this statement is also correct.
Conclusion
After analyzing all statements, we find that all of the statements provided are correct. Therefore, the answer to the question, "Which of the following statements is wrong?" is:
Answer: None of these (all statements are correct).
---
Statement: Ozone is produced in the upper stratosphere by the action of UV rays on oxygen.
Statement: Ozone can oxidize Sulphur dioxide present in the atmosphere to Sulphur trioxide.
Statement: Ozone hole is thinning of the ozone layer present in the stratosphere.
Statement: None of these.
After analyzing all statements, we find that all of the statements provided are correct. Therefore, the answer to the question, "Which of the following statements is wrong?" is:
Topper's Solved these Questions
- ENVIRONMENTAL CHEMISTRYBook:VK JAISWAL ENGLISHChapter:ENVIRONMENTAL CHEMISTRYExercise:Level 3
Explore 4 Videos
- ENVIRONMENTAL CHEMISTRYBook:VK JAISWAL ENGLISHChapter:ENVIRONMENTAL CHEMISTRYExercise:ONE OR MORE ANSWER IS/ARE CORRECT
Explore 8 Videos
- ENVIRONMENTAL CHEMISTRYBook:VK JAISWAL ENGLISHChapter:ENVIRONMENTAL CHEMISTRYExercise:ASSERTION-REASON TYPE QUESTIONS
Explore 14 Videos
- d-BLOCK ELEMENTSBook:VK JAISWAL ENGLISHChapter:d-BLOCK ELEMENTSExercise:SUBJECTIVE PROBLEMS
Explore 6 Videos
- METALLURGYBook:VK JAISWAL ENGLISHChapter:METALLURGYExercise:ASSERTION-REASON TYPE QUESTIONS
Explore 29 Videos
Similar Questions
When heathly earth's stratosphere contains a low concentration of ozone(O3) that absorbs potentially harmful ultraviolet (UV) radiations by the cycle shown below:
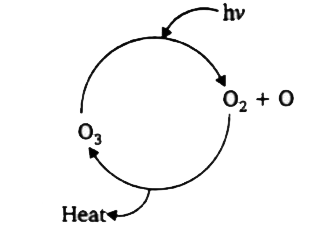
Chloroflouro carbon refrigerants, such as freon 12(CF2Cl2), are stable in lower atmosphere but in the stratosphere, they absorb high energy UV radiation to generate chlorine radicals.
CF2Cl2hv−−→CF2Cl+Cl
The presence of small number of chlorine radicals appears to lower ozone concentrations dramatically. The following reactions are all known to be exothermic (except the one requiring light) and to have high rate constant.
A:Cl−O−O−Clhv−−→O2+2Cl⋅
B:Cl⋅O+O→O2+Cl⋅
C:Cl⋅+O3→Cl−O⋅+O2
D:2Cl−O⋅→Cl−O−O−Cl
Q. Ozone has the ability to absorb
View SolutionWhich of the following conditions shows the polluted enviorment ?
View SolutionAssertion (A) Green house effect was observed in houses used to grow plants and these are made of green glass.
Reason (R) Green house name has been given because glass houses are made of green glass.
View SolutionCalculate the magnetic moment of a high-spin octahedral complex that has six electrons in 3d-orbitals
View SolutionAssertion : PbSiF6+H2SiF6+ gelatine is taken as electrolyte in electrolytic refining of lead.
Reason : Discharge potential of Pb2+ is less than H+.
View SolutionWhich of the following statements is wrong ?
View SolutionWhich of the following statements is/are wrong ?
View SolutionWhich of the following statements is wrong?
View SolutionWhich of the following statements is wrong ?
View SolutionWhich of the following statement is wrong .
View SolutionWhich of the following statements is wrong?
View SolutionWhich of the following statements is wrong ?
View SolutionWhich of the following statements is wrong ?
View SolutionWhich of the following statement is wrong ?
View SolutionWhich of the following statement is wrong?
View SolutionWhich of the following statement is wrong ?
View Solution
