Assertion: AgNO3 reacts with KCN to form white ppt. of AgCN. This white ppt. Disappears when excess KCN is added.
Reason: AgCN decomposes to form silver-carbide and evolve N2 gas.
If both assertion and reason are true and the reason is the correct explanation of assertion
If both assertion and reason are true and the reason is not the correct explanation of assertion
If assertion is false but reason is true
If assertion is true but the reason is false
The correct Answer is:C
Step by step video solution for Assertion: AgNO_(3) reacts with KCN to form white ppt. of AgCN. This white ppt. Disappears when excess KCN is added. Reason: AgCN decomposes to form silver-carbide and evolve N_(2) gas. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- d-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - d-BLOCK ELEMENTSEXERCISE - SUBJECTIVE PROBLEMS
6 Videos
- d-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - d-BLOCK ELEMENTSEXERCISE - MATCH THE COLUMN
3 Videos
- CO-ORDINATION COMPOUNDSBOOK - VK JAISWAL ENGLISHCHAPTER - CO-ORDINATION COMPOUNDSEXERCISE - SUBJECTIVE PROBLEMS
42 Videos
- ENVIRONMENTAL CHEMISTRYBOOK - VK JAISWAL ENGLISHCHAPTER - ENVIRONMENTAL CHEMISTRYEXERCISE - ASSERTION-REASON TYPE QUESTIONS
14 Videos
Similar Questions
Calculate the magnetic moment of a high-spin octahedral complex that has six electrons in 3d-orbitals
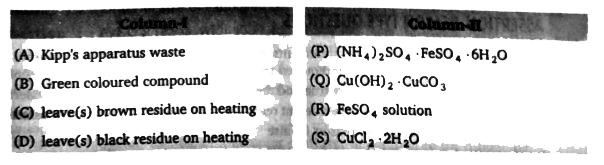
View SolutionEntries of Column-I are to be matched with entries of Coloumn-II. Each entry of Column-I may have the matching with one or more than one entries of Column-II.
View SolutionConsider the following compounds with their regular geometries.

Calculate value of (x+y−z)2, where x is total number of axial d-orbitals having zero nodal plane, y and z are total no. of non-axial and axial d-orbitals respectively each one having two nodal planes used in hybridisation of central atoms of compounds A to E.
View SolutionAssertion (A) Green house effect was observed in houses used to grow plants and these are made of green glass.
Reason (R) Green house name has been given because glass houses are made of green glass.
View SolutionHaloalkanes react with KCN to form alkyl cyanides as main product while AgCN forms isocyanides as the chief product. Explain.
View SolutionAccount for the following Chloromethane reacts with KCN to form ethanenitrile as the main product and wit AgCN to form methyl isocyanide as the chief product.
View SolutionAssertion: Haloalkanes react with KCN to form alkyl cyanides as main product while with AgCN form isocyanide as the main product.
Reason: KCN and AgCN, both are ionic compounds.
View SolutionHaloakanes react with KCN to form alkyl cyanides as main product while AgCN forms isocyanides as the chief product . Explain .
View SolutionOn adding AgNO_(3) to NaCl white ppt , occurs
View SolutionAssertion: AgNO_(3) reacts with KCN to form white ppt. of AgCN. This white ppt. Disappears when excess KCN is added. Reason: AgCN decomposes to form silver-carbide and evolve N_(2) gas.
View SolutionAssertion: AgNO3 reacts with KCNN to form white ppt. of AgCN. This white ppt. disappears when excess kCN is added.
Reason: AgCN decomposes to form silver-carbide and evolve N2 gas.
View Solutionऐल्किलसायनाइड की KCN से अभिक्रिया द्वारा मुख्य उत्पाद ऐल्किल सायनाइड बनता है, जबकि AgCN से अभिक्रिया करने पर आइसोसायनाइड मुख्य उत्पाद होता है | कारण स्पष्ट कीजिए |
View SolutionAssertion: AgNO_(3) reacts with KCN to form white ppt. of AgCN. This white ppt. Disappears when excess KCN is added. Reason: AgCN decomposes to form silver-carbide and evolve N_(2) gas.
View SolutionAccount for following : Chloromethane reacts with KCN to form ethane nitrile as main product and with AgCN to form methyl isocyanide as chief product.
View SolutionHaloalkanes react with KCN to form alkyl cyanides as main product while AgCN forms isocyanides as the chief product. Explain.
View SolutionHaloalkanes react with KCN to form alkyl cyanides as main product while AgCN forms isocyanides as the chief product. Explain.
View Solution

