A solid mixture of AgCl ad K2Cr2O7 is heated with conc. H2SO4 and produces:
greenish yellow gas
colourless gas
red coloured gas
no gas
The correct Answer is:D
To solve the problem of what happens when a solid mixture of AgCl and K₂Cr₂O₇ is heated with concentrated H₂SO₄, we can follow these steps:
Step 1: Identify the Reactants
The reactants in this reaction are:
- Silver chloride (AgCl)
- Potassium dichromate (K₂Cr₂O₇)
- Concentrated sulfuric acid (H₂SO₄)
Step 2: Understand the Reaction Type
This reaction is a double displacement reaction (also known as a metathesis reaction). In this type of reaction, the ions in the reactants exchange partners to form new compounds.
Step 3: Write the Reaction
When AgCl reacts with K₂Cr₂O₇ in the presence of concentrated H₂SO₄, the following products are formed:
- Silver dichromate (Ag₂Cr₂O₇)
- Potassium chloride (KCl)
The balanced chemical equation for this reaction can be written as:
2AgCl+K2Cr2O7+4H2SO4→Ag2Cr2O7+2KCl+4HCl+2H2O
Step 4: Analyze the Products
- Silver dichromate (Ag₂Cr₂O₇): This is a solid that may appear as a red or orange compound.
- Potassium chloride (KCl): This is a soluble salt that remains in the solution.
- Hydrochloric acid (HCl): This is a gas that is released during the reaction.
- Water (H₂O): This is also produced as a byproduct.
Step 5: Conclusion
When a solid mixture of AgCl and K₂Cr₂O₇ is heated with concentrated H₂SO₄, the products formed include silver dichromate, potassium chloride, hydrochloric acid, and water. Notably, hydrochloric acid is released as a gas during the reaction.
Final Answer
The reaction produces silver dichromate, potassium chloride, hydrochloric acid (gas), and water.
---
The reactants in this reaction are:
- Silver chloride (AgCl)
- Potassium dichromate (K₂Cr₂O₇)
- Concentrated sulfuric acid (H₂SO₄)
This reaction is a double displacement reaction (also known as a metathesis reaction). In this type of reaction, the ions in the reactants exchange partners to form new compounds.
When AgCl reacts with K₂Cr₂O₇ in the presence of concentrated H₂SO₄, the following products are formed:
- Silver dichromate (Ag₂Cr₂O₇)
- Potassium chloride (KCl)
2AgCl+K2Cr2O7+4H2SO4→Ag2Cr2O7+2KCl+4HCl+2H2O
- Silver dichromate (Ag₂Cr₂O₇): This is a solid that may appear as a red or orange compound.
- Potassium chloride (KCl): This is a soluble salt that remains in the solution.
- Hydrochloric acid (HCl): This is a gas that is released during the reaction.
- Water (H₂O): This is also produced as a byproduct.
When a solid mixture of AgCl and K₂Cr₂O₇ is heated with concentrated H₂SO₄, the products formed include silver dichromate, potassium chloride, hydrochloric acid, and water. Notably, hydrochloric acid is released as a gas during the reaction.
The reaction produces silver dichromate, potassium chloride, hydrochloric acid (gas), and water.
Topper's Solved these Questions
- QUALITATIVE INORGANIC ANALYSISBook:VK JAISWAL ENGLISHChapter:QUALITATIVE INORGANIC ANALYSISExercise:LEVEL 2
Explore 76 Videos
- QUALITATIVE INORGANIC ANALYSISBook:VK JAISWAL ENGLISHChapter:QUALITATIVE INORGANIC ANALYSISExercise:LEVEL 3
Explore 44 Videos
- PERIODIC PROPERTIESBook:VK JAISWAL ENGLISHChapter:PERIODIC PROPERTIESExercise:MATCH THE COLUMN
Explore 11 Videos
- s-BLOCK ELEMENTSBook:VK JAISWAL ENGLISHChapter:s-BLOCK ELEMENTSExercise:SUBJECTIVE PROBLEMS
Explore 2 Videos
Similar Questions
When a reagent (X) reacts with Fe3+ salt solution turnsred due to the formation of a compound (Y). This reagent cause no change in colour with Fe2+ salt solution. Compound (X) and (Y) are respectively:
View SolutionA colourless inorganic compound (A) imparts a green colour to the flame. Its solution gives a white ppt. (B) with H2SO4. When heated with K2Cr2O7 and conc. H2SO4, a brown red vapour/gas (C) is formed. The gas/ vapour when passed through aqueous NaOH solution, it turns into a yellow solution (D) which forms yellow precipitate (E) with CH3COOH and (CH3COO)2Pb. with reference to above information, answe the following questions.
Q. The colourless inorganic compound (A) is:
View Solution
View Solution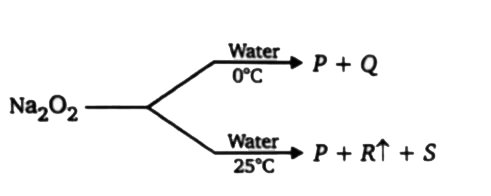
Calculate sum of bond order between same bonded atoms in Q and R compounds.
View SolutionThe reaction of NaCl and K_(2)Cr_(2)O_(7) mixture with conc. H_(2)SO_(4) result in the formation of:
View SolutionA mixture of K2Cr2O7 and conc. H2SO4 forms
View SolutionWhen K_(2)Cr_(2)O_(7) is heated with conc. H_(2)SO_(4) and soluble chloride such as KCl :
View SolutionOn heating K_(2)Cr_(2)O_(7) with NaCl and conc. H_(2)SO_(4) , the gas liberated is
View SolutionWhich of the following compounds is formed when a mixture of K_(2)Cr_(2) O_(7) and KCl is heated with conc. H_(2)SO_(4) ?
View SolutionThe reddish brown gas produced by heating KCl with solid K2Cr2O7 and conc. H2SO4 is
View SolutionA solid mixture of AgCl ad K_(2)Cr_(2)O_(7) is heated with conc. H_(2)SO_(4) and produces:
View SolutionOn heating a mixture of NaCl, K_(2)Cr_(2)O_(7) and conc. H_(2)SO_(4) which of the following is formed ?
View SolutionWhen KCl is heated with conc. H_(2)SO_(4) and solid K_(2)Cr_(2)O_(7), we get
View SolutionOn heating a metal chloride with K2Cr2O7 and conc. H2SO4, the gas evolved is :
View SolutionWhich of the following statement is not correct when a mixture of NaCl and K2Cr2O7 is warmed with conc. H2SO4 ?
View Solution
