(A) light blue coloured compound on heating will convert into black (B) which reacts with glucose gives red compound (C) and (A) reacts with ammonium hdyroxide in excess in presence of ammonium sulphate give blue compound (D). What is (A), (B), (C) and (D)?
[Cu(NH3)4]SO4,CuO,Cu2O,CuSO4
CuSO4,CuO,Cu2O,Cu(OH)2
Cu(OH)2,Cu2O,CuO,[Cu(NH3)4]SO4
Cu(OH)2,CuO,Cu2O,[Cu(NH3)4]SO4
The correct Answer is:D
Step by step video solution for (A) light blue coloured compound on heating will convert into black (B) which reacts with glucose gives red compound (C) and (A) reacts with ammonium hdyroxide in excess in presence of ammonium sulphate give blue compound (D). What is (A), (B), (C) and (D)? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- QUALITATIVE INORGANIC ANALYSISBook:VK JAISWAL ENGLISHChapter:QUALITATIVE INORGANIC ANALYSISExercise:LEVEL 3
Explore 44 Videos
- QUALITATIVE INORGANIC ANALYSISBook:VK JAISWAL ENGLISHChapter:QUALITATIVE INORGANIC ANALYSISExercise:ONE OR MORE ANSWER IS/ARE CORRECT
Explore 41 Videos
- QUALITATIVE INORGANIC ANALYSISBook:VK JAISWAL ENGLISHChapter:QUALITATIVE INORGANIC ANALYSISExercise:SUBJECTIVE PROBLEMS
Explore 3 Videos
- PERIODIC PROPERTIESBook:VK JAISWAL ENGLISHChapter:PERIODIC PROPERTIESExercise:MATCH THE COLUMN
Explore 11 Videos
- s-BLOCK ELEMENTSBook:VK JAISWAL ENGLISHChapter:s-BLOCK ELEMENTSExercise:SUBJECTIVE PROBLEMS
Explore 2 Videos
Similar Questions
A colourless inorganic compound (A) imparts a green colour to the flame. Its solution gives a white ppt. (B) with H2SO4. When heated with K2Cr2O7 and conc. H2SO4, a brown red vapour/gas (C) is formed. The gas/ vapour when passed through aqueous NaOH solution, it turns into a yellow solution (D) which forms yellow precipitate (E) with CH3COOH and (CH3COO)2Pb. with reference to above information, answe the following questions.
Q. The colourless inorganic compound (A) is:
View SolutionBasic radical(s) which can not be identified by borax bead test:
View SolutionFind number of basic radicals among the following cations, which can form soluble complex on adding excess of NH4 solution.
Cd2+(aq.),Pb2+(aq.),Ni2+(aq.),Mn2+(aq.),Zn2+(aq.),Ag+(aq.),Hg2+(aq.),Fe3+(aq.),Mg2+(aq.)
View Solution
View Solution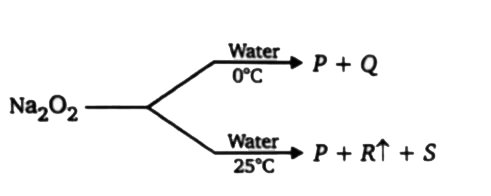
Calculate sum of bond order between same bonded atoms in Q and R compounds.
View Solution(a). An ore (A) on roasting with sodium carbonate and lime in the presence of air gives two compounds (B) and (C). (b). The solution of (B) in conc. HCl on treatment with potassiu ferroyanide gives a blue colour or precipitate of compound (D).
View SolutionAn inorganic compound 'A' on heating with solution of KOH , gives a gas B and the solution of compound C. gas B on ignition with excess of air gives a compound D and water. Copper sulphate gives a black precipitate on passing through its solution. White precipitate E is obtained on reaction of C with copper sulphates solution. Compound D is:
View SolutionBuff coloured cyanide compound (A) reacts with excess of KCN in presence of an oxidising agent gives (B). Which d orbitals is/are participating in hybridisation of complex (B)?
View SolutionA nitraite on acid hydrolysis gives compound A, which reacts with thionyl chloride to give compound B. Benzene reacts with compound B in presence of anhydrous AlCl3 to give compound C. The compound B reacts with ethyl alcohol to give ester D. Identify the compounds A,B C and D and write the equations.
View SolutionAn organic compound A upon reacting with NH_3 gives B. On heating B gives C. C in presence of KOH reacts with Br_2 to given CH_3CH_2NH_2 . A is :
View SolutionBenzonitirle (A) reacts with stannous chloride in the presence of dil HCl to give a reduction product 'B' . Compound 'B' on acid hydrolysis gives compound 'C' . Identify compound 'B' and 'C' . What is the nitration product of compound 'C' ?
View Solution(A) light blue coloured compound on heating will convert into black (B) which reacts with glucose gives red compound (C) and (A) reacts with ammonium hdyroxide in excess in presence of ammonium sulphate give blue compound (D). What is (A), (B), (C) and (D)?
View SolutionIdentify the compound (A) which is a universal solvent. Compound A reacts with chlorine gas to give B and C. Compound A dissolves in an ionic compound of silicon to give compound D. Identify A and write the equations involved in the formation of B, C and D.
View SolutionAn element belongs to group 1 and period 3 reacts with chlorine gas to form A. (A) reacts with ammonium bicarbonate to give compounds (B) and (C). Compound period is beryllium (A). (B) is then heated to give compound (D). Identify A, B, C and D and write the respective equations.
View SolutionA compound reacts withs excess nitric acid and ammonium molibdate to give a yellow prepcipitate and with AgN3 a red preciptate. The compound si -
View Solution
